KMUCAT Paper 1 2025
Quiz-summary
0 of 100 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
Information
- Kindly keep some rough pages and pen with you for rough work
- The Explanations of all the MCQ’s will be provided to you with Your Right & Wrong Answers on completion of this Test.
- Bismillah & Darood Sharif Phr k, Start your Test/Quiz, and Attempt it with Full Concentration.
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Results
0 of 100 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 points, (0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
-
Thanks for the Participation.
1) Take Screenshot of your Result. (For Your Record)
2) You can Click On View Questions (Button)
For MCQ’s Explanations
and for your Right / Wrong Answers
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
- Answered
- Review
-
Question 1 of 100
1. Question
1 pointsThe species which are becoming near to extinction are called:
Correct
Explanation: B
• Wild species: These are species living in their natural habitats without human domestication. The term “wild” does not necessarily mean they are at risk of extinction.
• Endangered species: These are species that are facing a very high risk of extinction in the near future. According to IUCN (International Union for Conservation of Nature), endangered species are just one step away from becoming extinct if not protected.
• Threatened species: This is a broader category used by IUCN, which includes endangered, vulnerable, and rare species. It means species are at risk of becoming endangered in the near future, but not necessarily on the verge right now.
• None: Not correct, because we do have proper terms in biology for such species.
Correct Answer: B. Endangered
Detailed Explanation
• The question specifically says: “becoming near to extinction”.
• This exact definition matches Endangered species, because they are species at risk of vanishing completely soon if no conservation action is taken.
• For example: Bengal tiger, snow leopard, and blackbuck in Pakistan/India are considered endangered.
• If the question had been about species that may become endangered in the future, the better answer would have been Threatened.
• But since it asks about species nearing extinction, the precise key is Endangered.
Final Key: B. EndangeredIncorrect
Explanation: B
• Wild species: These are species living in their natural habitats without human domestication. The term “wild” does not necessarily mean they are at risk of extinction.
• Endangered species: These are species that are facing a very high risk of extinction in the near future. According to IUCN (International Union for Conservation of Nature), endangered species are just one step away from becoming extinct if not protected.
• Threatened species: This is a broader category used by IUCN, which includes endangered, vulnerable, and rare species. It means species are at risk of becoming endangered in the near future, but not necessarily on the verge right now.
• None: Not correct, because we do have proper terms in biology for such species.
Correct Answer: B. Endangered
Detailed Explanation
• The question specifically says: “becoming near to extinction”.
• This exact definition matches Endangered species, because they are species at risk of vanishing completely soon if no conservation action is taken.
• For example: Bengal tiger, snow leopard, and blackbuck in Pakistan/India are considered endangered.
• If the question had been about species that may become endangered in the future, the better answer would have been Threatened.
• But since it asks about species nearing extinction, the precise key is Endangered.
Final Key: B. Endangered -
Question 2 of 100
2. Question
1 pointsWhat number comes next in the sequence 2, 6, 12, 20, 30?
Correct
Explanation: B
Step 1: Look for a pattern
• First differences:
6 – 2 = 4
12 – 6 = 6
20 – 12 = 8
30 – 20 = 10
So the differences are 4, 6, 8, 10 (increasing by 2 each time).
Step 2: Find the next difference
• The next difference will be 12.
So,
30 + 12 = 42
Correct Answer: B. 42
The sequence is based on the formula:
n(n+1),n=1,2,3,4,5,…
• For n = 1 → 1×2 = 2
• For n = 2 → 2×3 = 6
• For n = 3 → 3×4 = 12
• For n = 4 → 4×5 = 20
• For n = 5 → 5×6 = 30
• For n = 6 → 6×7 = 42
Thus, the next term is 42.
Final Key: B. 42.Incorrect
Explanation: B
Step 1: Look for a pattern
• First differences:
6 – 2 = 4
12 – 6 = 6
20 – 12 = 8
30 – 20 = 10
So the differences are 4, 6, 8, 10 (increasing by 2 each time).
Step 2: Find the next difference
• The next difference will be 12.
So,
30 + 12 = 42
Correct Answer: B. 42
The sequence is based on the formula:
n(n+1),n=1,2,3,4,5,…
• For n = 1 → 1×2 = 2
• For n = 2 → 2×3 = 6
• For n = 3 → 3×4 = 12
• For n = 4 → 4×5 = 20
• For n = 5 → 5×6 = 30
• For n = 6 → 6×7 = 42
Thus, the next term is 42.
Final Key: B. 42. -
Question 3 of 100
3. Question
1 pointsWhat is a figure of speech in which two unlike things are compared using “like” or “as”?
Correct
Explanation: C
A simile is a figure of speech that explicitly compares two unlike things using “like” or “as.” It helps create vivid imagery or clarify an idea.
Examples
• “Her smile shines like the sun.”
• “He is as brave as a lion.”
• “The car moved like a rocket.”
How it differs from the other options
• Metaphor (A): Implied comparison without “like/as.”
“Time is a thief.” (not “like a thief”)
• Personification (B): Gives human qualities to nonhuman things.
“The wind whispered through the trees.”
• Hyperbole (D): Exaggeration for effect.
“I’ve told you a million times.”
Quick exam tip
Not every sentence with “as” is a simile. It must compare two different kinds of things for imagery.
• Not a simile: “Ali is as tall as Bilal.” (same kind of thing: two people)
• Simile: “Ali is as busy as a bee.” (person vs. bee)
Final Key: C. Simile.Incorrect
Explanation: C
A simile is a figure of speech that explicitly compares two unlike things using “like” or “as.” It helps create vivid imagery or clarify an idea.
Examples
• “Her smile shines like the sun.”
• “He is as brave as a lion.”
• “The car moved like a rocket.”
How it differs from the other options
• Metaphor (A): Implied comparison without “like/as.”
“Time is a thief.” (not “like a thief”)
• Personification (B): Gives human qualities to nonhuman things.
“The wind whispered through the trees.”
• Hyperbole (D): Exaggeration for effect.
“I’ve told you a million times.”
Quick exam tip
Not every sentence with “as” is a simile. It must compare two different kinds of things for imagery.
• Not a simile: “Ali is as tall as Bilal.” (same kind of thing: two people)
• Simile: “Ali is as busy as a bee.” (person vs. bee)
Final Key: C. Simile. -
Question 4 of 100
4. Question
1 pointsWhat letter comes in the following series? B, D, G, K, P
Correct
Explanation: D
Convert letters to positions in the alphabet:
• B=2, D=4, G=7, K=11, P=16
Look at the differences:
• 2 → 4 (+2)
• 4 → 7 (+3)
• 7 → 11 (+4)
• 11 → 16 (+5)
The step pattern increases by 1 each time: +2, +3, +4, +5, so next is +6.
Compute next term:
• 16 + 6 = 22 → the 22nd letter is V.
Final Key: D. VIncorrect
Explanation: D
Convert letters to positions in the alphabet:
• B=2, D=4, G=7, K=11, P=16
Look at the differences:
• 2 → 4 (+2)
• 4 → 7 (+3)
• 7 → 11 (+4)
• 11 → 16 (+5)
The step pattern increases by 1 each time: +2, +3, +4, +5, so next is +6.
Compute next term:
• 16 + 6 = 22 → the 22nd letter is V.
Final Key: D. V -
Question 5 of 100
5. Question
1 pointsBRASS is to metal as SPARROW is to:
Correct
Explanation: B
The analogy is category–member (type-of) relationship:
• BRASS is a type of metal.
• SPARROW is a type of bird.
Why the others are wrong
• A. Animal: Too broad—sparrow is an animal, but the parallel to “metal” is the immediate category, which is “bird.”
• C. Wings: Part–whole relation, not a category.
• D. Fly: An action/verb (or an insect), not a category.
Final Key: B. BirdIncorrect
Explanation: B
The analogy is category–member (type-of) relationship:
• BRASS is a type of metal.
• SPARROW is a type of bird.
Why the others are wrong
• A. Animal: Too broad—sparrow is an animal, but the parallel to “metal” is the immediate category, which is “bird.”
• C. Wings: Part–whole relation, not a category.
• D. Fly: An action/verb (or an insect), not a category.
Final Key: B. Bird -
Question 6 of 100
6. Question
1 pointsWhat is the name for a group of words that contains a subject and a verb but does not express a complete thought?
Correct
Explanation: B
Step-by-step Explanation
• Phrase (A): A group of words without a subject-verb combination.
Example: in the park, the big dog, running fast.
• Clause (B): A group of words with a subject and a verb.
Independent clause: expresses a complete thought → She is reading a book.
Dependent clause: does not express a complete thought → Because she was tired…
This matches the question.
Sentence (C): An independent clause (or more) that expresses a complete thought.
Example: He plays football.
• Paragraph (D): A collection of related sentences.
Final Key: B. Clause.Incorrect
Explanation: B
Step-by-step Explanation
• Phrase (A): A group of words without a subject-verb combination.
Example: in the park, the big dog, running fast.
• Clause (B): A group of words with a subject and a verb.
Independent clause: expresses a complete thought → She is reading a book.
Dependent clause: does not express a complete thought → Because she was tired…
This matches the question.
Sentence (C): An independent clause (or more) that expresses a complete thought.
Example: He plays football.
• Paragraph (D): A collection of related sentences.
Final Key: B. Clause. -
Question 7 of 100
7. Question
1 pointsIf five boxes of soap weigh 75kg and each box when empty weighs 3kg, how much is the weight of the soap?
Correct
Explanation: C
Step 1: Total given weight
• 5 boxes (with soap inside) weigh = 75 kg
Step 2: Weight of empty boxes
• Each empty box = 3 kg
• 5 boxes empty weight = 5 × 3 = 15 kg
Step 3: Weight of soap alone
• Total weight − empty box weight = 75 − 15 = 60 kg
Correct Answer: C. 60 kg
Key point: Always separate the container’s weight from the content’s weight.Incorrect
Explanation: C
Step 1: Total given weight
• 5 boxes (with soap inside) weigh = 75 kg
Step 2: Weight of empty boxes
• Each empty box = 3 kg
• 5 boxes empty weight = 5 × 3 = 15 kg
Step 3: Weight of soap alone
• Total weight − empty box weight = 75 − 15 = 60 kg
Correct Answer: C. 60 kg
Key point: Always separate the container’s weight from the content’s weight. -
Question 8 of 100
8. Question
1 pointsWhat is the term for a sentence that gives a command or makes a request?
Correct
Explanation: D
Step-by-step Explanation
• A. Statement (Declarative): A sentence that gives information or states a fact.
Example: The sun rises in the east.
• B. Question (Interrogative): A sentence that asks something.
Example: Where are you going?
• C. Exclamation (Exclamatory): A sentence that shows strong feelings, often ending with an exclamation mark.
Example: What a beautiful day!
• D. Imperative: A sentence that gives a command, instruction, or request.
Example: Close the door. / Please help me.
The question clearly asks for the type that gives a command or makes a request, so the correct answer is Imperative.
Final Key: D. Imperative.Incorrect
Explanation: D
Step-by-step Explanation
• A. Statement (Declarative): A sentence that gives information or states a fact.
Example: The sun rises in the east.
• B. Question (Interrogative): A sentence that asks something.
Example: Where are you going?
• C. Exclamation (Exclamatory): A sentence that shows strong feelings, often ending with an exclamation mark.
Example: What a beautiful day!
• D. Imperative: A sentence that gives a command, instruction, or request.
Example: Close the door. / Please help me.
The question clearly asks for the type that gives a command or makes a request, so the correct answer is Imperative.
Final Key: D. Imperative. -
Question 9 of 100
9. Question
1 pointsIf Akbar is younger than Mehmood and Ayaz, and Ayaz is older than Mehmood, and Zubair is younger than Ayaz, who is the oldest?
Correct
Explanation: A
From the statements:
• Akbar is younger than Mehmood and Ayaz → Akbar can’t be oldest.
• Ayaz is older than Mehmood → Mehmood can’t be oldest.
• Zubair is younger than Ayaz → Zubair can’t be oldest.
Thus, Ayaz is older than Mehmood and Zubair, and also older than Akbar (since Akbar is younger than Ayaz).
So the oldest is Ayaz.Incorrect
Explanation: A
From the statements:
• Akbar is younger than Mehmood and Ayaz → Akbar can’t be oldest.
• Ayaz is older than Mehmood → Mehmood can’t be oldest.
• Zubair is younger than Ayaz → Zubair can’t be oldest.
Thus, Ayaz is older than Mehmood and Zubair, and also older than Akbar (since Akbar is younger than Ayaz).
So the oldest is Ayaz. -
Question 10 of 100
10. Question
1 pointsWhat is the term for a sentence that expresses strong emotion or surprise?
Correct
Explanation: C
• A. Statement (Declarative): A sentence that simply gives information or states a fact.
Example: The sky is blue.
• B. Question (Interrogative): A sentence that asks something.
Example: Where are you going?
• C. Exclamation (Exclamatory): A sentence that shows strong emotion, excitement, or surprise, usually ending with an exclamation mark (!).
Example: What a wonderful day it is!
This matches the question.
• D. Imperative: A sentence that gives a command or request.
Example: Close the door.
Final Key: C. Exclamation.Incorrect
Explanation: C
• A. Statement (Declarative): A sentence that simply gives information or states a fact.
Example: The sky is blue.
• B. Question (Interrogative): A sentence that asks something.
Example: Where are you going?
• C. Exclamation (Exclamatory): A sentence that shows strong emotion, excitement, or surprise, usually ending with an exclamation mark (!).
Example: What a wonderful day it is!
This matches the question.
• D. Imperative: A sentence that gives a command or request.
Example: Close the door.
Final Key: C. Exclamation. -
Question 11 of 100
11. Question
1 pointsThe dimension of work is like the dimensions of:
Correct
Explanation: B
Step 1: Recall the formula for Work
W = F⋅d
• Force F = ma = MLT−2
• Distance d = L
So,
[W] = MLT−2⋅ L = ML2T−2
Step 2: Check each option
• A. Impulse
J = F ⋅ t = (MLT−2)(T) = MLT−1 Not the same.
• B. Torque
τ = F ⋅ r = (MLT−2)(L) = ML2T−2 =
Same as work.
• C. Power
P = W/t = ML2T−2 /T = ML2T−3
Different.
• D. Angular momentum
L = mvr = (M)(LT−1)(L) = ML2T−1
Different.
Correct Answer: B. Torque
Key point: Work and Torque have the same dimensions (ML²T⁻²), but they represent different physical quantities.Incorrect
Explanation: B
Step 1: Recall the formula for Work
W = F⋅d
• Force F = ma = MLT−2
• Distance d = L
So,
[W] = MLT−2⋅ L = ML2T−2
Step 2: Check each option
• A. Impulse
J = F ⋅ t = (MLT−2)(T) = MLT−1 Not the same.
• B. Torque
τ = F ⋅ r = (MLT−2)(L) = ML2T−2 =
Same as work.
• C. Power
P = W/t = ML2T−2 /T = ML2T−3
Different.
• D. Angular momentum
L = mvr = (M)(LT−1)(L) = ML2T−1
Different.
Correct Answer: B. Torque
Key point: Work and Torque have the same dimensions (ML²T⁻²), but they represent different physical quantities. -
Question 12 of 100
12. Question
1 pointsThe time rate of change of magnetic flux has the exact dimensions as that of:
Correct
Explanation: D
Step 1: Recall Faraday’s law of electromagnetic induction
Induced emf (E)=−dΦ/dt
• Here, Φ = magnetic flux = B⋅A
• dΦ/dt is the time rate of change of magnetic flux, which equals emf.
So, the time rate of change of flux has the same dimensions as potential difference (voltage).
Step 2: Dimension check
• Potential difference (V):
V = WQ = ML2T−2/IT = ML2T−3I−1
• dΦ/dt (rate of change of flux):
Magnetic flux = B⋅A = (Magnetic induction) × (Area).
Dimension of B = MT−2I−1.
Area = L2.
So flux = ML2T−2I−1.
Divide by time (T):
dΦ/dt = ML2T−3I−1
This is exactly the dimension of potential difference.
Correct Answer: D. Potential differenceIncorrect
Explanation: D
Step 1: Recall Faraday’s law of electromagnetic induction
Induced emf (E)=−dΦ/dt
• Here, Φ = magnetic flux = B⋅A
• dΦ/dt is the time rate of change of magnetic flux, which equals emf.
So, the time rate of change of flux has the same dimensions as potential difference (voltage).
Step 2: Dimension check
• Potential difference (V):
V = WQ = ML2T−2/IT = ML2T−3I−1
• dΦ/dt (rate of change of flux):
Magnetic flux = B⋅A = (Magnetic induction) × (Area).
Dimension of B = MT−2I−1.
Area = L2.
So flux = ML2T−2I−1.
Divide by time (T):
dΦ/dt = ML2T−3I−1
This is exactly the dimension of potential difference.
Correct Answer: D. Potential difference -
Question 13 of 100
13. Question
1 pointsThe magnitude of i·(j × k) is:
Correct
Explanation: A
Step 1: Recall the standard unit vectors
• i , j , k are mutually perpendicular unit vectors along the x, y, and z axes.
Step 2: Compute the cross product
j × k = i
Step 3: Take the dot product with i
i⋅(j × k) = i ⋅ I = 1
Step 4: Magnitude
Since it is already a scalar value = 1, the magnitude is also 1.
Correct Answer: A. 1
Key Idea: This is the definition of the scalar triple product a ⋅ (b × c). For unit vectors in cyclic order (i, j, k), it equals +1.Incorrect
Explanation: A
Step 1: Recall the standard unit vectors
• i , j , k are mutually perpendicular unit vectors along the x, y, and z axes.
Step 2: Compute the cross product
j × k = i
Step 3: Take the dot product with i
i⋅(j × k) = i ⋅ I = 1
Step 4: Magnitude
Since it is already a scalar value = 1, the magnitude is also 1.
Correct Answer: A. 1
Key Idea: This is the definition of the scalar triple product a ⋅ (b × c). For unit vectors in cyclic order (i, j, k), it equals +1. -
Question 14 of 100
14. Question
1 pointsA mass accelerates uniformly when the resultant force acting on it is:
Correct
Explanation: B
Step 1: Recall Newton’s Second Law
F = ma
• If F = 0, then a = 0 → the body moves with uniform velocity (no acceleration).
• If F is constant and not zero, then a = F/ma = constant → the body accelerates uniformly.
• If F increases uniformly with time, then acceleration also increases with time → non-uniform acceleration.
Step 2: Check each option
• A. Zero → No acceleration, uniform velocity. (not right)
• B. Constant but not zero → Produces uniform acceleration. (right)
• C. Increasing uniformly with respect to time → Produces increasing (non-uniform) acceleration. (not right)
• D. Both A and C → Both wrong. (not right)
Correct Answer: B. Constant but not zero
Key Idea: A body accelerates uniformly only when the net force is constant and non-zero.Incorrect
Explanation: B
Step 1: Recall Newton’s Second Law
F = ma
• If F = 0, then a = 0 → the body moves with uniform velocity (no acceleration).
• If F is constant and not zero, then a = F/ma = constant → the body accelerates uniformly.
• If F increases uniformly with time, then acceleration also increases with time → non-uniform acceleration.
Step 2: Check each option
• A. Zero → No acceleration, uniform velocity. (not right)
• B. Constant but not zero → Produces uniform acceleration. (right)
• C. Increasing uniformly with respect to time → Produces increasing (non-uniform) acceleration. (not right)
• D. Both A and C → Both wrong. (not right)
Correct Answer: B. Constant but not zero
Key Idea: A body accelerates uniformly only when the net force is constant and non-zero. -
Question 15 of 100
15. Question
1 pointsA stone thrown horizontally from the top of a building follows a path that is:
Correct
Explanation: D
• A stone thrown horizontally has:
Horizontal motion: Constant velocity (no horizontal acceleration, ignoring air resistance).
Vertical motion: Uniform acceleration due to gravity.
• When these two motions combine (constant horizontal + uniformly accelerated vertical), the resultant trajectory is a parabola.
Why other options are wrong
A. Circular: Needs centripetal force toward a center. Not the case here.
B. Made of two straight line segments: Motion is smooth and curved, not broken lines.
C. Hyperbolic: Hyperbola doesn’t describe projectile paths under uniform gravity.
Final Key: D. Parabolic.Incorrect
Explanation: D
• A stone thrown horizontally has:
Horizontal motion: Constant velocity (no horizontal acceleration, ignoring air resistance).
Vertical motion: Uniform acceleration due to gravity.
• When these two motions combine (constant horizontal + uniformly accelerated vertical), the resultant trajectory is a parabola.
Why other options are wrong
A. Circular: Needs centripetal force toward a center. Not the case here.
B. Made of two straight line segments: Motion is smooth and curved, not broken lines.
C. Hyperbolic: Hyperbola doesn’t describe projectile paths under uniform gravity.
Final Key: D. Parabolic. -
Question 16 of 100
16. Question
1 pointsA hunter aims at a bird perched on a branch. The bird drops from rest at the instant the bullet is fired. Neglect air resistance. To hit the bird, the hunter should aim:
Correct
Explanation: B
Both bullet and bird fall with the same acceleration g starting at t=0, so the straight line from muzzle to the bird’s initial position remains the line of impact.Incorrect
Explanation: B
Both bullet and bird fall with the same acceleration g starting at t=0, so the straight line from muzzle to the bird’s initial position remains the line of impact. -
Question 17 of 100
17. Question
1 pointsThe heat energy dissipated by a 40-watt bulb in one hour is:
Correct
Explanation: C
Step 1: Formula for energy
The electrical (or heat) energy dissipated by a bulb is given by:
E = P × t
where
• PPP = power in watts (J/s)
• ttt = time in seconds
Step 2: Convert given values
• Power = 40 W = 40 J/s
• Time = 1 hour = 1 × 60 × 60=3600 s
Step 3: Calculate energy
E = 40 × 3600 = 144000 J
Correct Answer: C. 144000 J
Key point: Always convert hours into seconds when using SI units.Incorrect
Explanation: C
Step 1: Formula for energy
The electrical (or heat) energy dissipated by a bulb is given by:
E = P × t
where
• PPP = power in watts (J/s)
• ttt = time in seconds
Step 2: Convert given values
• Power = 40 W = 40 J/s
• Time = 1 hour = 1 × 60 × 60=3600 s
Step 3: Calculate energy
E = 40 × 3600 = 144000 J
Correct Answer: C. 144000 J
Key point: Always convert hours into seconds when using SI units. -
Question 18 of 100
18. Question
1 pointsWhen a body moves against the force of friction on a horizontal plane, the work done by the body is:
Correct
Explanation: B
• Work done is defined as:
W = F⋅d⋅cosθ
where F is the applied force, d is the displacement, and θ is the angle between force and displacement.
• On a horizontal plane with friction present, the body moves forward because of the applied force in the same direction as displacement.
• Even though friction opposes the motion, the work done by the body (applied force) to overcome friction is still positive, since the applied force and displacement are in the same direction (θ = 0∘ ⇒ cosθ = 1).
• If we asked about work done by friction, that would be negative, because friction acts opposite to displacement.
Why other options are wrong
• A. Negative: That’s the case for friction’s work, not the body’s applied work.
• C. Zero: Work is zero only if there is no displacement or force perpendicular to motion. Not true here.
• D. Maximum and positive: “Maximum” is not necessarily true — it depends on the magnitude of force.
Final Key: B. Positive
Key concept: Work done by the applied force overcoming friction is positive, while work done by friction is negativeIncorrect
Explanation: B
• Work done is defined as:
W = F⋅d⋅cosθ
where F is the applied force, d is the displacement, and θ is the angle between force and displacement.
• On a horizontal plane with friction present, the body moves forward because of the applied force in the same direction as displacement.
• Even though friction opposes the motion, the work done by the body (applied force) to overcome friction is still positive, since the applied force and displacement are in the same direction (θ = 0∘ ⇒ cosθ = 1).
• If we asked about work done by friction, that would be negative, because friction acts opposite to displacement.
Why other options are wrong
• A. Negative: That’s the case for friction’s work, not the body’s applied work.
• C. Zero: Work is zero only if there is no displacement or force perpendicular to motion. Not true here.
• D. Maximum and positive: “Maximum” is not necessarily true — it depends on the magnitude of force.
Final Key: B. Positive
Key concept: Work done by the applied force overcoming friction is positive, while work done by friction is negative -
Question 19 of 100
19. Question
1 pointsWhich of the following type of force do not work?
Correct
Explanation: D
• Work done formula:
W = F⋅d⋅cosθ
where F is the force, d is displacement, and θ is the angle between them.
Check each option:
• A. Elastic force:
Acts along the direction of displacement (e.g., stretching a spring).
Does positive or negative work.
• B. Frictional force:
Always opposes displacement.
Does negative work.
• C. Gravitational force:
When a body falls, displacement is along force of gravity.
Does positive work.
• D. Centripetal force:
Acts toward the center of the circle, while displacement is tangent to the circle.
Angle between them = 90∘
W = F⋅d⋅cos90∘== 0.
Does no work.
Final Key: D. Centripetal force
Key concept: Any force perpendicular to displacement does zero work.Incorrect
Explanation: D
• Work done formula:
W = F⋅d⋅cosθ
where F is the force, d is displacement, and θ is the angle between them.
Check each option:
• A. Elastic force:
Acts along the direction of displacement (e.g., stretching a spring).
Does positive or negative work.
• B. Frictional force:
Always opposes displacement.
Does negative work.
• C. Gravitational force:
When a body falls, displacement is along force of gravity.
Does positive work.
• D. Centripetal force:
Acts toward the center of the circle, while displacement is tangent to the circle.
Angle between them = 90∘
W = F⋅d⋅cos90∘== 0.
Does no work.
Final Key: D. Centripetal force
Key concept: Any force perpendicular to displacement does zero work. -
Question 20 of 100
20. Question
1 pointsWhen sound waves move from one medium to another, the quantity which remains the same is:
Correct
Explanation: B
When a sound wave crosses from one medium to another (e.g., air → water):
• Speed (C): Changes, because wave speed depends on the medium’s density and elasticity.
• Wavelength (A): Also changes, since λ = v/f. If speed changes and frequency is fixed, wavelength must change.
• Intensity (D): Can change due to reflection, refraction, or absorption at the boundary.
• Frequency (B): Remains unchanged, because frequency is determined by the source of vibration, not by the medium.
Final Key: B. Frequency
Key concept: At a boundary, frequency stays constant, while wavelength and speed adjust to the new medium.Incorrect
Explanation: B
When a sound wave crosses from one medium to another (e.g., air → water):
• Speed (C): Changes, because wave speed depends on the medium’s density and elasticity.
• Wavelength (A): Also changes, since λ = v/f. If speed changes and frequency is fixed, wavelength must change.
• Intensity (D): Can change due to reflection, refraction, or absorption at the boundary.
• Frequency (B): Remains unchanged, because frequency is determined by the source of vibration, not by the medium.
Final Key: B. Frequency
Key concept: At a boundary, frequency stays constant, while wavelength and speed adjust to the new medium. -
Question 21 of 100
21. Question
1 pointsWhat is correct for all transverse waves?
Correct
Explanation: B
A. They will involve the oscillation of the atoms
Not true for all.
In mechanical transverse waves (like waves on a string or water waves), atoms/particles oscillate perpendicular to propagation.
But in electromagnetic transverse waves, oscillating electric and magnetic fields exist, not atoms.
B. They can all be polarized
True.
Polarization is the restriction of wave vibrations to a single plane.
Only transverse waves can be polarized because their oscillations are perpendicular to the direction of propagation.
Longitudinal waves (like sound in air) cannot be polarized.
C. They can travel through a vacuum
Not true for all.
Electromagnetic transverse waves can travel through a vacuum.
Mechanical transverse waves (like water waves, waves on a rope) cannot travel through a vacuum (need a medium).
D. Both A & C are correct
Wrong, since both A and C are not universally true.
Final Key: B. They can all be polarized
Key Concept: Polarization is the unique property of transverse waves and does not apply to longitudinal waves.Incorrect
Explanation: B
A. They will involve the oscillation of the atoms
Not true for all.
In mechanical transverse waves (like waves on a string or water waves), atoms/particles oscillate perpendicular to propagation.
But in electromagnetic transverse waves, oscillating electric and magnetic fields exist, not atoms.
B. They can all be polarized
True.
Polarization is the restriction of wave vibrations to a single plane.
Only transverse waves can be polarized because their oscillations are perpendicular to the direction of propagation.
Longitudinal waves (like sound in air) cannot be polarized.
C. They can travel through a vacuum
Not true for all.
Electromagnetic transverse waves can travel through a vacuum.
Mechanical transverse waves (like water waves, waves on a rope) cannot travel through a vacuum (need a medium).
D. Both A & C are correct
Wrong, since both A and C are not universally true.
Final Key: B. They can all be polarized
Key Concept: Polarization is the unique property of transverse waves and does not apply to longitudinal waves. -
Question 22 of 100
22. Question
1 pointsA 30 cm long string with one end clamped and the other free to move transversely is vibrating in its second harmonic. The wavelength of the constituent traveling waves is:
Correct
Explanation: C
Step 1: Boundary condition
• String length = 30 cm
• One end clamped (fixed), other end free.
This is a closed–open system, similar to an organ pipe with one closed end and one open end.
In such systems:
• Only odd harmonics are possible.
• n-th harmonic has:
L = (2n−1)λ/4
Step 2: Second harmonic in closed–open string
• Fundamental (1st harmonic): L = λ/4
• 2nd harmonic (which is actually the 3rd harmonic of a full open–open case):
L = 3λ/4
Step 3: Plug in values
30 = 3λ/4
λ = 30 × 4/3 = 40 cm.
Correct Answer: C. 40 cm
Key Concept: For a string (or air column) with one fixed and one free end, only odd harmonics occur, and the wavelength for the 2nd harmonic is linked by L = 3λ/4.Incorrect
Explanation: C
Step 1: Boundary condition
• String length = 30 cm
• One end clamped (fixed), other end free.
This is a closed–open system, similar to an organ pipe with one closed end and one open end.
In such systems:
• Only odd harmonics are possible.
• n-th harmonic has:
L = (2n−1)λ/4
Step 2: Second harmonic in closed–open string
• Fundamental (1st harmonic): L = λ/4
• 2nd harmonic (which is actually the 3rd harmonic of a full open–open case):
L = 3λ/4
Step 3: Plug in values
30 = 3λ/4
λ = 30 × 4/3 = 40 cm.
Correct Answer: C. 40 cm
Key Concept: For a string (or air column) with one fixed and one free end, only odd harmonics occur, and the wavelength for the 2nd harmonic is linked by L = 3λ/4. -
Question 23 of 100
23. Question
1 pointsFor all irreversible processes, the entropy of the universe _____________.
Correct
Explanation: D
• Entropy is a measure of disorder/randomness of a system.
• According to the Second Law of Thermodynamics:
→ For a reversible process, the change in entropy of the system + surroundings = 0.
→ For an irreversible process, the total entropy of the system + surroundings always increases.
Check options
• A. Decreases (not true) → This never happens naturally; it would violate the second law.
• B. Remains constant (not true) → True only for reversible processes, not irreversible.
C. Zero (not true) → That’s the entropy change of the universe in a reversible cycle, not of an irreversible process.
D. Increases (True) → Always true for irreversible processes.
Final Key: D. Increases
Key Concept:
Irreversible processes are spontaneous and always cause an increase in entropy, which aligns with the “arrow of time.”Incorrect
Explanation: D
• Entropy is a measure of disorder/randomness of a system.
• According to the Second Law of Thermodynamics:
→ For a reversible process, the change in entropy of the system + surroundings = 0.
→ For an irreversible process, the total entropy of the system + surroundings always increases.
Check options
• A. Decreases (not true) → This never happens naturally; it would violate the second law.
• B. Remains constant (not true) → True only for reversible processes, not irreversible.
C. Zero (not true) → That’s the entropy change of the universe in a reversible cycle, not of an irreversible process.
D. Increases (True) → Always true for irreversible processes.
Final Key: D. Increases
Key Concept:
Irreversible processes are spontaneous and always cause an increase in entropy, which aligns with the “arrow of time.” -
Question 24 of 100
24. Question
1 pointsThe temperature scale which is independent of the nature of the working substance is:
Correct
Explanation: D
• A. Celsius scale
Based on the properties of mercury (or other materials) — depends on the working substance.
• B. Fahrenheit scale
Also depends on material properties (originally based on alcohol, water, etc.).
• C. Centigrade scale
This is just another name for Celsius scale, also substance-dependent.
• D. Thermodynamic scale
Derived directly from the Second Law of Thermodynamics.
Does not depend on the property of any particular material (like mercury, alcohol, gas).
Absolute zero on this scale is a true physical limit.
Example: Kelvin scale is a thermodynamic scale.
Final Key: D. Thermodynamic scale
Key Concept: The thermodynamic (Kelvin) scale is universal and independent of the working substance, unlike practical scales (Celsius, Fahrenheit).Incorrect
Explanation: D
• A. Celsius scale
Based on the properties of mercury (or other materials) — depends on the working substance.
• B. Fahrenheit scale
Also depends on material properties (originally based on alcohol, water, etc.).
• C. Centigrade scale
This is just another name for Celsius scale, also substance-dependent.
• D. Thermodynamic scale
Derived directly from the Second Law of Thermodynamics.
Does not depend on the property of any particular material (like mercury, alcohol, gas).
Absolute zero on this scale is a true physical limit.
Example: Kelvin scale is a thermodynamic scale.
Final Key: D. Thermodynamic scale
Key Concept: The thermodynamic (Kelvin) scale is universal and independent of the working substance, unlike practical scales (Celsius, Fahrenheit). -
Question 25 of 100
25. Question
1 pointsIf the temperature of the source of heat increases, the efficiency of a Carnot engine:
Correct
Explanation: A
The efficiency of a Carnot engine is given by:
η = 1 – TC/TH
where
• TH = absolute temperature of the hot source,
• TC = absolute temperature of the cold sink.
Effect of increasing THT_HTH
• If the temperature of the hot source increases while the cold sink temperature remains constant:
• The fraction TC/TH becomes smaller.
• Therefore, η=1 – TC/TH becomes larger.
Thus, efficiency increases.
Why other options are wrong
B. Decreases: Opposite effect, not correct.
C. Remains constant: Only if both TH and TC change in the same ratio.
D. None of the above: Not needed since option A is correct.
Final Key: A. Increases
Key Concept: A Carnot engine is more efficient when the source is hotter or the sink is colder, because the temperature difference between them determines efficiency.Incorrect
Explanation: A
The efficiency of a Carnot engine is given by:
η = 1 – TC/TH
where
• TH = absolute temperature of the hot source,
• TC = absolute temperature of the cold sink.
Effect of increasing THT_HTH
• If the temperature of the hot source increases while the cold sink temperature remains constant:
• The fraction TC/TH becomes smaller.
• Therefore, η=1 – TC/TH becomes larger.
Thus, efficiency increases.
Why other options are wrong
B. Decreases: Opposite effect, not correct.
C. Remains constant: Only if both TH and TC change in the same ratio.
D. None of the above: Not needed since option A is correct.
Final Key: A. Increases
Key Concept: A Carnot engine is more efficient when the source is hotter or the sink is colder, because the temperature difference between them determines efficiency. -
Question 26 of 100
26. Question
1 pointsThe negative gradient of electric potential is also called:
Correct
Explanation: B
• Electric potential V(r) is a scalar field.
• The electric field E is related to potential by
E=− ∇V
i.e., the negative gradient of the potential. The gradient points toward the direction of greatest increase of V; the minus sign makes E point from higher to lower potential (the way a positive charge “rolls downhill”).
• In 1-D: Ex=− dV/dx. Units: V/m = N/C.
Quick intuition: Between parallel plates with potential difference ΔV and separation d, the field magnitude is E = ΔV/d and points from the positive plate (higher V) to the negative plate (lower V), consistent with E = −∇V.
Why the others are wrong
• A. Potential energy: U = qV. The force is the negative gradient of potential energy: F = −∇U. Dividing by q gives E = −∇V, not −∇U.
• C. “Destine potential energy”: not a standard physics term; not applicable here.
• D. Electron volt (eV): a unit of energy, equal to the work done on one electron through a potential difference of 1 V
(1 eV = 1.602 × 10−19 J); it is not a field or gradient.Incorrect
Explanation: B
• Electric potential V(r) is a scalar field.
• The electric field E is related to potential by
E=− ∇V
i.e., the negative gradient of the potential. The gradient points toward the direction of greatest increase of V; the minus sign makes E point from higher to lower potential (the way a positive charge “rolls downhill”).
• In 1-D: Ex=− dV/dx. Units: V/m = N/C.
Quick intuition: Between parallel plates with potential difference ΔV and separation d, the field magnitude is E = ΔV/d and points from the positive plate (higher V) to the negative plate (lower V), consistent with E = −∇V.
Why the others are wrong
• A. Potential energy: U = qV. The force is the negative gradient of potential energy: F = −∇U. Dividing by q gives E = −∇V, not −∇U.
• C. “Destine potential energy”: not a standard physics term; not applicable here.
• D. Electron volt (eV): a unit of energy, equal to the work done on one electron through a potential difference of 1 V
(1 eV = 1.602 × 10−19 J); it is not a field or gradient. -
Question 27 of 100
27. Question
1 pointsTwo metallic conductors have the same resistivity. These conductors can be differentiated from the values of their:
Correct
Explanation: A
Resistivity (ρ) is a fundamental property of a material that is the same for all conductors made of the same substance at a given temperature.
R = ρ L/A
Here, R is resistance, L is length, A is cross-sectional area.
• If two metallic conductors have the same resistivity, that means they are made of the same material (at a given reference temperature).
• But, even for the same resistivity, conductors can be differentiated by their temperature coefficient of resistance (α), which tells us how much resistance changes with temperature:
RT = R0 (1 + αΔT).
Why other options are wrong:
B. Resistance: Depends on dimensions (length and area). Two conductors of the same material but different shapes can have different resistances. But if dimensions are unknown, resistance alone is not a fundamental distinguishing property.
C. Conductance: Reciprocal of resistance, so also depends on shape/size, not just material.
D. Conductivity (σ = 1/ρ): Since resistivity is the same, conductivity is also the same.
Key Point: The temperature coefficient of resistance is the property that allows us to differentiate between two metallic conductors with the same resistivity.Incorrect
Explanation: A
Resistivity (ρ) is a fundamental property of a material that is the same for all conductors made of the same substance at a given temperature.
R = ρ L/A
Here, R is resistance, L is length, A is cross-sectional area.
• If two metallic conductors have the same resistivity, that means they are made of the same material (at a given reference temperature).
• But, even for the same resistivity, conductors can be differentiated by their temperature coefficient of resistance (α), which tells us how much resistance changes with temperature:
RT = R0 (1 + αΔT).
Why other options are wrong:
B. Resistance: Depends on dimensions (length and area). Two conductors of the same material but different shapes can have different resistances. But if dimensions are unknown, resistance alone is not a fundamental distinguishing property.
C. Conductance: Reciprocal of resistance, so also depends on shape/size, not just material.
D. Conductivity (σ = 1/ρ): Since resistivity is the same, conductivity is also the same.
Key Point: The temperature coefficient of resistance is the property that allows us to differentiate between two metallic conductors with the same resistivity. -
Question 28 of 100
28. Question
1 pointsIn transistor, the emitter–base junction is:
Correct
Explanation: B
• A transistor has two PN junctions:
1. Emitter–Base (E–B) junction
2. Collector–Base (C–B) junction
• For a transistor to work in its active region (the normal mode of amplification):
Emitter–Base junction is forward biased → allows majority carriers to be injected from the emitter into the base.
Collector–Base junction is reverse biased → collects those carriers efficiently.
• If the E–B junction were reverse biased, almost no carriers would cross, and the transistor could not function as an amplifier.
Why other options are wrong:
A. Reverse biased: This applies to the collector–base junction, not the emitter–base junction.
C. Neutral: Junctions cannot be neutral in operation; they are either forward or reverse biased.
D. None of these: Incorrect since a clear correct option exists.
Key Answer: B. Forward biasedIncorrect
Explanation: B
• A transistor has two PN junctions:
1. Emitter–Base (E–B) junction
2. Collector–Base (C–B) junction
• For a transistor to work in its active region (the normal mode of amplification):
Emitter–Base junction is forward biased → allows majority carriers to be injected from the emitter into the base.
Collector–Base junction is reverse biased → collects those carriers efficiently.
• If the E–B junction were reverse biased, almost no carriers would cross, and the transistor could not function as an amplifier.
Why other options are wrong:
A. Reverse biased: This applies to the collector–base junction, not the emitter–base junction.
C. Neutral: Junctions cannot be neutral in operation; they are either forward or reverse biased.
D. None of these: Incorrect since a clear correct option exists.
Key Answer: B. Forward biased -
Question 29 of 100
29. Question
1 pointsIn Einstein’s universe, what is the fourth dimension?
Correct
Explanation: B
• In Einstein’s theory of Relativity, the universe is described as a four-dimensional continuum called space-time.
• The first three dimensions are the familiar spatial coordinates: length, width, and height.
• The fourth dimension is time, which is inseparably linked with space.
• Events in the universe are described by four coordinates: (x, y, z, t).
Why the others are wrong:
A. Speed: It is a derived quantity, not a fundamental dimension.
C. Energy: A form of physical quantity, not a dimension.
D. Space: Already represented by the first three dimensions.
Final Answer: B. Time.Incorrect
Explanation: B
• In Einstein’s theory of Relativity, the universe is described as a four-dimensional continuum called space-time.
• The first three dimensions are the familiar spatial coordinates: length, width, and height.
• The fourth dimension is time, which is inseparably linked with space.
• Events in the universe are described by four coordinates: (x, y, z, t).
Why the others are wrong:
A. Speed: It is a derived quantity, not a fundamental dimension.
C. Energy: A form of physical quantity, not a dimension.
D. Space: Already represented by the first three dimensions.
Final Answer: B. Time. -
Question 30 of 100
30. Question
1 pointsThe excited state which persists for an unusually longer period of time is called:
Correct
Explanation: C
• Excited state: When an atom absorbs energy, electrons jump to higher energy levels. Usually, they drop back to the ground state very quickly (within nanoseconds).
• Metastable state: A special type of excited state in which the electron remains trapped for a comparatively longer time before returning to a lower energy level.
→ Lifetimes can be microseconds to milliseconds (much longer than ordinary excited states).
→ This property is crucial in lasers, where metastable states allow a population inversion to occur.
Why the others are wrong:
A. Ground state: The lowest, most stable energy level — not an excited state.
B. Ionized state: When an electron is completely removed from the atom — not a long-lived excited state.
D. Ordinary excited state: Very short-lived compared to metastable states.
Final Answer: C. Metastable state.Incorrect
Explanation: C
• Excited state: When an atom absorbs energy, electrons jump to higher energy levels. Usually, they drop back to the ground state very quickly (within nanoseconds).
• Metastable state: A special type of excited state in which the electron remains trapped for a comparatively longer time before returning to a lower energy level.
→ Lifetimes can be microseconds to milliseconds (much longer than ordinary excited states).
→ This property is crucial in lasers, where metastable states allow a population inversion to occur.
Why the others are wrong:
A. Ground state: The lowest, most stable energy level — not an excited state.
B. Ionized state: When an electron is completely removed from the atom — not a long-lived excited state.
D. Ordinary excited state: Very short-lived compared to metastable states.
Final Answer: C. Metastable state. -
Question 31 of 100
31. Question
1 pointsOne atom of sodium is:
Correct
Explanation: D
• Avogadro’s number (NA) = 6.022 × 1023 atoms per mole.
By definition:
1 mole of sodium = 6.022 × 1023 atoms.
• Therefore, 1 atom of sodium corresponds to:
Number of moles = 1/NA moles.
Why other options are wrong:
A. 23 g: That’s the molar mass of sodium (1 mole = 23 g), not the mass of one atom.
B. Moles: Vague and incorrect — a single atom cannot be “moles.”
C. Positively charged: A neutral sodium atom is not charged. A sodium ion (Na+) is positively charged, but the question asks about an atom.
Final Answer: D. 1/NA.Incorrect
Explanation: D
• Avogadro’s number (NA) = 6.022 × 1023 atoms per mole.
By definition:
1 mole of sodium = 6.022 × 1023 atoms.
• Therefore, 1 atom of sodium corresponds to:
Number of moles = 1/NA moles.
Why other options are wrong:
A. 23 g: That’s the molar mass of sodium (1 mole = 23 g), not the mass of one atom.
B. Moles: Vague and incorrect — a single atom cannot be “moles.”
C. Positively charged: A neutral sodium atom is not charged. A sodium ion (Na+) is positively charged, but the question asks about an atom.
Final Answer: D. 1/NA. -
Question 32 of 100
32. Question
1 pointsOne gram atom of chlorine is equal to:
Correct
Explanation: D
1. Meaning of gram atom
A gram atom means 1 mole of atoms of an element.
Thus, 1 gram atom of chlorine = 1 mole of chlorine atoms.
2. Atomic mass of chlorine
Atomic mass of chlorine = 35.5 u.
Hence, 1 mole of chlorine atoms has a mass of 35.5 g.
3. Molecular mass vs. atomic mass
Note: Chlorine exists naturally as a diatomic molecule Cl2, with molecular mass = 71.
But the term gram atom refers to a single atom, not the molecule.
Therefore, 1 gram atom of chlorine = 35.5 g, not 71 g.
Why options are correct/incorrect:
A. One mole of chlorine atoms (definition of gram atom).
B. 35.5 g of chlorine atom (mass of 1 mole of Cl atoms).
C. 71 g (this is the gram molecular mass of Cl2, not a gram atom).
Final Answer: D. Both A & B.Incorrect
Explanation: D
1. Meaning of gram atom
A gram atom means 1 mole of atoms of an element.
Thus, 1 gram atom of chlorine = 1 mole of chlorine atoms.
2. Atomic mass of chlorine
Atomic mass of chlorine = 35.5 u.
Hence, 1 mole of chlorine atoms has a mass of 35.5 g.
3. Molecular mass vs. atomic mass
Note: Chlorine exists naturally as a diatomic molecule Cl2, with molecular mass = 71.
But the term gram atom refers to a single atom, not the molecule.
Therefore, 1 gram atom of chlorine = 35.5 g, not 71 g.
Why options are correct/incorrect:
A. One mole of chlorine atoms (definition of gram atom).
B. 35.5 g of chlorine atom (mass of 1 mole of Cl atoms).
C. 71 g (this is the gram molecular mass of Cl2, not a gram atom).
Final Answer: D. Both A & B. -
Question 33 of 100
33. Question
1 pointsTotal charge on an electron is approximately:
A. 1.6 × 10–19 C
B. 9.1×10–31 kg
C. 1.6 × 10–19 J
D. 3.0 × 108 m/sCorrect
Explanation: A
• The charge of an electron is a fundamental constant:
e = −1.602 × 10−19 C
(negative sign shows the charge is negative).
The options given:
A. 1.6 × 10−19 C: Correct, this is the magnitude of electron charge.
B. 9.1 × 10−31 kg: This is the mass of an electron, not its charge.
C. 1.6 × 10−19 J: This is the energy equivalent of 1 eV, not charge.
D. 3.0 × 108 m/s: This is the speed of light, unrelated to electron charge.Incorrect
Explanation: A
• The charge of an electron is a fundamental constant:
e = −1.602 × 10−19 C
(negative sign shows the charge is negative).
The options given:
A. 1.6 × 10−19 C: Correct, this is the magnitude of electron charge.
B. 9.1 × 10−31 kg: This is the mass of an electron, not its charge.
C. 1.6 × 10−19 J: This is the energy equivalent of 1 eV, not charge.
D. 3.0 × 108 m/s: This is the speed of light, unrelated to electron charge. -
Question 34 of 100
34. Question
1 pointsIf the highest occupied principal quantum number of an atom is n=4, what is the maximum number of electrons the atom can accommodate when shells n=1 to 4 are completely filled?
Correct
Explanation: B
Capacity by shell = 2n^2. Sum 2 + 8 + 18 + 32 = 60.Incorrect
Explanation: B
Capacity by shell = 2n^2. Sum 2 + 8 + 18 + 32 = 60. -
Question 35 of 100
35. Question
1 pointsVelocity of an electron will be maximum in the:
Correct
Explanation: A
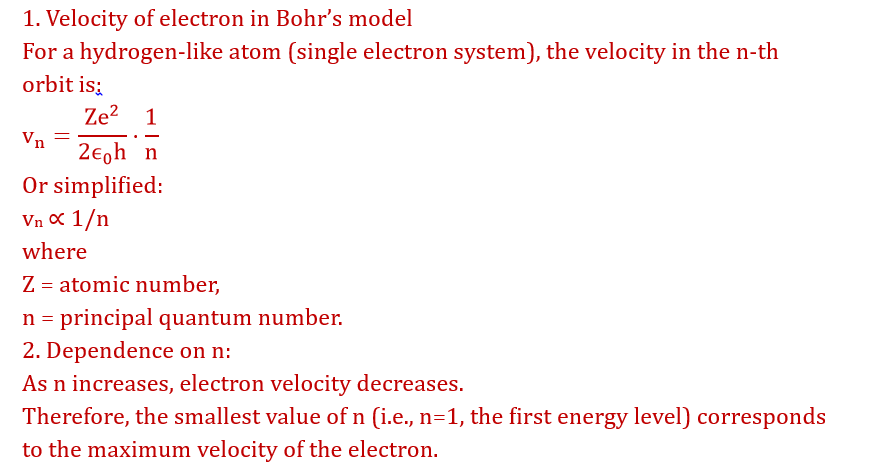
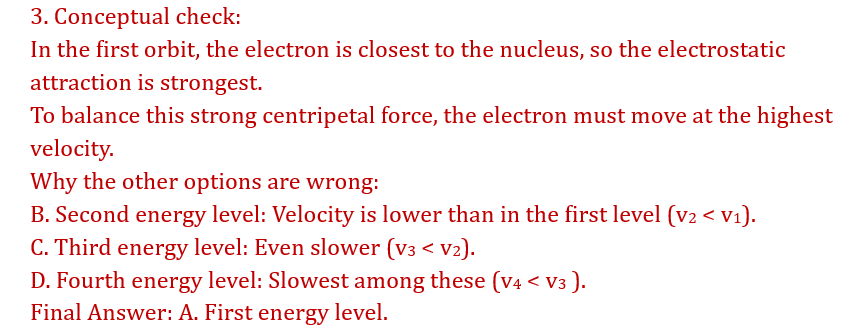 Incorrect
Incorrect
Explanation: A


-
Question 36 of 100
36. Question
1 pointsA container with a porous wall is filled with one mole each of He, H2, N2, and CO2. Which one will take least time to effuse?
A. He
B. H2
C. N2
D. O2Correct
Explanation: B

 Incorrect
Incorrect
Explanation: B


-
Question 37 of 100
37. Question
1 pointsThe behavior of a gas is non-ideal at:
Correct
Explanation: B
1. Ideal Gas Assumptions
• Gas molecules have negligible volume.
• No intermolecular forces exist.
• These assumptions are valid only when:
→ Gas molecules are far apart (low pressure).
→ Molecules move fast (high temperature), so attractions don’t matter.
2. When gases deviate (non-ideal behavior):
• At low temperature: Molecules move slowly → intermolecular attractions become significant.
• At high pressure: Molecules are compressed close together → finite molecular volume is no longer negligible.
2. Conclusion:
• Under low T and high P, the assumptions of the ideal gas law fail, and real gases show maximum deviation (non-ideal behavior).
Why others are wrong:
• A. High T, low P: Conditions close to ideal gas behavior.
• C. Low T, low P: Attractions may exist, but low pressure reduces collisions, so deviation is not maximum.
• D. High T, high P: High T reduces attraction, but high P increases repulsion due to volume; effect is less than B.
Final Answer: B. Low T, High P.Incorrect
Explanation: B
1. Ideal Gas Assumptions
• Gas molecules have negligible volume.
• No intermolecular forces exist.
• These assumptions are valid only when:
→ Gas molecules are far apart (low pressure).
→ Molecules move fast (high temperature), so attractions don’t matter.
2. When gases deviate (non-ideal behavior):
• At low temperature: Molecules move slowly → intermolecular attractions become significant.
• At high pressure: Molecules are compressed close together → finite molecular volume is no longer negligible.
2. Conclusion:
• Under low T and high P, the assumptions of the ideal gas law fail, and real gases show maximum deviation (non-ideal behavior).
Why others are wrong:
• A. High T, low P: Conditions close to ideal gas behavior.
• C. Low T, low P: Attractions may exist, but low pressure reduces collisions, so deviation is not maximum.
• D. High T, high P: High T reduces attraction, but high P increases repulsion due to volume; effect is less than B.
Final Answer: B. Low T, High P. -
Question 38 of 100
38. Question
1 pointsWhich element has the strongest Van der Waals forces?
A. H2
B. Cl2
C. O2
D. N2Correct
Explanation: B
1. Van der Waals forces (London dispersion forces):
These are weak intermolecular forces arising due to temporary dipoles.
Their strength increases with:
• Molecular mass (heavier molecules → stronger forces).
• Size of electron cloud / polarizability (more easily distorted clouds → stronger forces).
2. Molecular masses of the given elements:
H2 → 2 g/mol
N2 → 28 g/mol
O2 → 32 g/mol
Cl2 → 71 g/mol
3. Comparison:
Cl2 is the heaviest molecule with the largest electron cloud, so it has the strongest Van der Waals forces.
This is why Cl2 is a liquid at room temperature, while H2, N2, and O2 are gases under the same conditions.
Why others are wrong:
A. H2: Very light, weakest Van der Waals forces.
C. O2: Stronger than N2 and H2 but weaker than Cl2.
D. N2: Lighter than O2 and Cl2, so weaker forces.
Final Answer: B. Cl₂Incorrect
Explanation: B
1. Van der Waals forces (London dispersion forces):
These are weak intermolecular forces arising due to temporary dipoles.
Their strength increases with:
• Molecular mass (heavier molecules → stronger forces).
• Size of electron cloud / polarizability (more easily distorted clouds → stronger forces).
2. Molecular masses of the given elements:
H2 → 2 g/mol
N2 → 28 g/mol
O2 → 32 g/mol
Cl2 → 71 g/mol
3. Comparison:
Cl2 is the heaviest molecule with the largest electron cloud, so it has the strongest Van der Waals forces.
This is why Cl2 is a liquid at room temperature, while H2, N2, and O2 are gases under the same conditions.
Why others are wrong:
A. H2: Very light, weakest Van der Waals forces.
C. O2: Stronger than N2 and H2 but weaker than Cl2.
D. N2: Lighter than O2 and Cl2, so weaker forces.
Final Answer: B. Cl₂ -
Question 39 of 100
39. Question
1 pointsEqual moles of H₂, O₂ and Cl₂ are in a vessel with a small pinhole. Rank their effusion rates (fastest → slowest).
A. H₂ > O₂ > Cl₂
B. H₂ > Cl₂ > O₂
C. O₂ > H₂ > Cl₂
D. Cl₂ > O₂ > H₂Correct
Explanation: A
By Graham’s law (r ∝ 1/√M), lower molar mass effuses faster.Incorrect
Explanation: A
By Graham’s law (r ∝ 1/√M), lower molar mass effuses faster. -
Question 40 of 100
40. Question
1 pointsWhich intermolecular force primarily explains why hydrogen peroxide (H₂O₂) is a liquid at room temperature?
Correct
Explanation: C
O–H groups enable strong hydrogen bonding, elevating the boiling point.Incorrect
Explanation: C
O–H groups enable strong hydrogen bonding, elevating the boiling point. -
Question 41 of 100
41. Question
1 pointsAll of the following are liquid crystals except:
Correct
Explanation: D
1. Liquid crystals (mesophases):
• Substances that have properties between solids (ordered) and liquids (fluid).
• They show anisotropy (direction-dependent properties) even in the liquid phase.
• Examples: soap solutions, DNA, and some cholesterol derivatives.
2. Examples given in the options:
• A. Soap solution: Forms micelles and shows lyotropic liquid crystalline phases.
• B. DNA: A biomolecule that exhibits liquid crystalline order under certain conditions.
• C. Cholesteryl formate: A derivative of cholesterol; well-known thermotropic liquid crystal used in liquid crystal displays (LCDs).
• D. Engine oil: An ordinary liquid, composed of hydrocarbons, does not form liquid crystalline phases.
2. Conclusion:
• All except engine oil exhibit liquid crystal behavior.
Final Answer: D. Engine oil.Incorrect
Explanation: D
1. Liquid crystals (mesophases):
• Substances that have properties between solids (ordered) and liquids (fluid).
• They show anisotropy (direction-dependent properties) even in the liquid phase.
• Examples: soap solutions, DNA, and some cholesterol derivatives.
2. Examples given in the options:
• A. Soap solution: Forms micelles and shows lyotropic liquid crystalline phases.
• B. DNA: A biomolecule that exhibits liquid crystalline order under certain conditions.
• C. Cholesteryl formate: A derivative of cholesterol; well-known thermotropic liquid crystal used in liquid crystal displays (LCDs).
• D. Engine oil: An ordinary liquid, composed of hydrocarbons, does not form liquid crystalline phases.
2. Conclusion:
• All except engine oil exhibit liquid crystal behavior.
Final Answer: D. Engine oil. -
Question 42 of 100
42. Question
1 pointsCrystals with lattice points occupied by metal ions are:
Correct
Explanation: D
1. Types of crystals based on lattice points:
• Ionic crystals: Lattice points occupied by positive and negative ions (e.g., NaCl, KBr).
• Covalent crystals (network solids): Lattice points occupied by atoms connected through covalent bonds (e.g., diamond, quartz).
• Molecular crystals: Lattice points occupied by neutral molecules held by Van der Waals forces or hydrogen bonding (e.g., ice, iodine).
• Metallic crystals: Lattice points occupied by metal ions, with a “sea of delocalized electrons” surrounding them (e.g., Cu, Fe, Na).
2. Given statement:
• “Crystals with lattice points occupied by metal ions” → definition of a metallic crystal.
Final Answer: D. Metallic.Incorrect
Explanation: D
1. Types of crystals based on lattice points:
• Ionic crystals: Lattice points occupied by positive and negative ions (e.g., NaCl, KBr).
• Covalent crystals (network solids): Lattice points occupied by atoms connected through covalent bonds (e.g., diamond, quartz).
• Molecular crystals: Lattice points occupied by neutral molecules held by Van der Waals forces or hydrogen bonding (e.g., ice, iodine).
• Metallic crystals: Lattice points occupied by metal ions, with a “sea of delocalized electrons” surrounding them (e.g., Cu, Fe, Na).
2. Given statement:
• “Crystals with lattice points occupied by metal ions” → definition of a metallic crystal.
Final Answer: D. Metallic. -
Question 43 of 100
43. Question
1 pointsAll of the following are covalent network crystals except:
A. SiO2
B. Graphite
C. Diamond
D. I2Correct
Explanation: D
1. Covalent network crystals (giant covalent solids):
• Atoms are bonded by strong covalent bonds throughout the crystal.
• Properties: very hard, high melting points, poor conductors (except graphite).
Examples:
• SiO2 (quartz): Network of Si–O covalent bonds.
• Diamond: Each carbon covalently bonded to 4 others in a tetrahedral network.
• Graphite: Each carbon bonded to 3 others in layers (covalent network in 2D, with weak forces between layers).
2. Molecular crystal:
• I2 (Iodine): Molecules held together by Van der Waals forces, not covalent bonding across the whole lattice.
• Hence, it is a molecular crystal, not a covalent network crystal.
Why others are correct examples:
• A. SiO2 → Covalent network crystal.
• B. Graphite → Covalent network in sheets.
• C. Diamond → 3D covalent network.
Final Answer: D. I2Incorrect
Explanation: D
1. Covalent network crystals (giant covalent solids):
• Atoms are bonded by strong covalent bonds throughout the crystal.
• Properties: very hard, high melting points, poor conductors (except graphite).
Examples:
• SiO2 (quartz): Network of Si–O covalent bonds.
• Diamond: Each carbon covalently bonded to 4 others in a tetrahedral network.
• Graphite: Each carbon bonded to 3 others in layers (covalent network in 2D, with weak forces between layers).
2. Molecular crystal:
• I2 (Iodine): Molecules held together by Van der Waals forces, not covalent bonding across the whole lattice.
• Hence, it is a molecular crystal, not a covalent network crystal.
Why others are correct examples:
• A. SiO2 → Covalent network crystal.
• B. Graphite → Covalent network in sheets.
• C. Diamond → 3D covalent network.
Final Answer: D. I2 -
Question 44 of 100
44. Question
1 points“X” is an imaginary plane which divides a crystal into mirror halves. The term is:
Correct
Explanation: B
1. Plane of symmetry:
• An imaginary plane that divides a crystal into two identical mirror-image halves.
• Each half is the reflection of the other.
• This matches the definition of “X” in the question.
2. Other symmetry elements (why incorrect here):
• A. Axis of rotation: An imaginary line about which the crystal can be rotated by a certain angle (like 2-fold, 3-fold) and still look the same.
• C. Center of symmetry: A point inside the crystal such that every face (or feature) has an identical one directly opposite to it.
• D. Axis of inversion: A line through the center such that inversion through this point maps every atom onto an equivalent position.
2. Conclusion:
• Since the question describes a plane dividing the crystal into mirror halves, the correct symmetry element is the plane of symmetry.Incorrect
Explanation: B
1. Plane of symmetry:
• An imaginary plane that divides a crystal into two identical mirror-image halves.
• Each half is the reflection of the other.
• This matches the definition of “X” in the question.
2. Other symmetry elements (why incorrect here):
• A. Axis of rotation: An imaginary line about which the crystal can be rotated by a certain angle (like 2-fold, 3-fold) and still look the same.
• C. Center of symmetry: A point inside the crystal such that every face (or feature) has an identical one directly opposite to it.
• D. Axis of inversion: A line through the center such that inversion through this point maps every atom onto an equivalent position.
2. Conclusion:
• Since the question describes a plane dividing the crystal into mirror halves, the correct symmetry element is the plane of symmetry. -
Question 45 of 100
45. Question
1 pointsIf 1 mole H₂ is mixed with 1 mole I₂ in empty container, with time rate of:
Correct
Explanation: D
1. Reaction considered:
H2(g)+I2(g) ⇌ 2HI(g)
2. Initial condition:
1 mole H2 and 1 mole I₂ are placed in an empty container.
At this instant, HI is absent, so the reverse reaction cannot occur yet.
3. With time:
As molecules collide, H2 + I2 → 2HI starts.
The rate of forward reaction increases initially because the reactants begin reacting and products (HI) start forming.
Only after some HI accumulates, the reverse reaction begins.
4. Equilibrium concept:
At the start: forward reaction dominates.
As time goes on: both forward and reverse occur.
At equilibrium: forward = reverse.
Why other options are wrong:
B. Forward & reverse remain unaffected: Wrong, rates change with time until equilibrium.
C. Reverse decreases: Reverse cannot decrease since it is zero initially and then increases.
D. Forward decreases: It decreases only after significant HI forms, not at the beginning.
Final Answer: A. Forward reaction increases.Incorrect
Explanation: D
1. Reaction considered:
H2(g)+I2(g) ⇌ 2HI(g)
2. Initial condition:
1 mole H2 and 1 mole I₂ are placed in an empty container.
At this instant, HI is absent, so the reverse reaction cannot occur yet.
3. With time:
As molecules collide, H2 + I2 → 2HI starts.
The rate of forward reaction increases initially because the reactants begin reacting and products (HI) start forming.
Only after some HI accumulates, the reverse reaction begins.
4. Equilibrium concept:
At the start: forward reaction dominates.
As time goes on: both forward and reverse occur.
At equilibrium: forward = reverse.
Why other options are wrong:
B. Forward & reverse remain unaffected: Wrong, rates change with time until equilibrium.
C. Reverse decreases: Reverse cannot decrease since it is zero initially and then increases.
D. Forward decreases: It decreases only after significant HI forms, not at the beginning.
Final Answer: A. Forward reaction increases. -
Question 46 of 100
46. Question
1 pointsA buffer is a solution which ___________________.
Correct
Explanation: B
Definition of a buffer:
• A buffer solution is one that resists changes in pH when small amounts of acid or base are added.
• It does this by having a weak acid and its conjugate base (or a weak base and its conjugate acid) present together.
Examples:
• Acidic buffer: CH3COOH + CH3COONa
• Basic buffer: NH4OH + NH₄Cl
Why other options are wrong:
A. Has constant pH: Not strictly true → the pH can change slightly, but the buffer resists large changes.
C. Strong acid + strong base: Their neutralization gives neutral salts (e.g., NaCl), which do not act as buffers.
D. Salts of strong acids/bases: Also do not act as buffers (e.g., NaCl solution has no buffering capacity).
Final Answer: B. Resists change in pH.Incorrect
Explanation: B
Definition of a buffer:
• A buffer solution is one that resists changes in pH when small amounts of acid or base are added.
• It does this by having a weak acid and its conjugate base (or a weak base and its conjugate acid) present together.
Examples:
• Acidic buffer: CH3COOH + CH3COONa
• Basic buffer: NH4OH + NH₄Cl
Why other options are wrong:
A. Has constant pH: Not strictly true → the pH can change slightly, but the buffer resists large changes.
C. Strong acid + strong base: Their neutralization gives neutral salts (e.g., NaCl), which do not act as buffers.
D. Salts of strong acids/bases: Also do not act as buffers (e.g., NaCl solution has no buffering capacity).
Final Answer: B. Resists change in pH. -
Question 47 of 100
47. Question
1 pointsEquilibrium constant expression in non-aqueous medium can be applied to:
Correct
Explanation: D
1. Equilibrium constant expression
The law of mass action and equilibrium constant expression can be applied to reactions in any medium (aqueous or non-aqueous).
But it is not applicable to non-reactive, non-electrolyte solutions where no equilibrium reaction occurs (like sugar in water).
2. Checking each option:
A. Aqueous sucrose: Sugar dissolves in water physically, no equilibrium reaction, so Keq expression is meaningless.
B. Aqueous table salt (NaCl): Strong electrolyte → dissociates completely, so no equilibrium is established (just ionic dissociation).
C. Aqueous fructose: Like sucrose, only physical dissolution, not chemical equilibrium.
D. Solution of kerosene: Kerosene is a non-aqueous medium. If a reaction is carried out in it (e.g., acid–base, complexation, organic reaction), then an equilibrium constant expression can be defined.
3. Conclusion:
Only non-aqueous reactive solutions like kerosene medium are valid cases for applying the equilibrium constant expression.
Final Answer: D. Solution of kerosene.Incorrect
Explanation: D
1. Equilibrium constant expression
The law of mass action and equilibrium constant expression can be applied to reactions in any medium (aqueous or non-aqueous).
But it is not applicable to non-reactive, non-electrolyte solutions where no equilibrium reaction occurs (like sugar in water).
2. Checking each option:
A. Aqueous sucrose: Sugar dissolves in water physically, no equilibrium reaction, so Keq expression is meaningless.
B. Aqueous table salt (NaCl): Strong electrolyte → dissociates completely, so no equilibrium is established (just ionic dissociation).
C. Aqueous fructose: Like sucrose, only physical dissolution, not chemical equilibrium.
D. Solution of kerosene: Kerosene is a non-aqueous medium. If a reaction is carried out in it (e.g., acid–base, complexation, organic reaction), then an equilibrium constant expression can be defined.
3. Conclusion:
Only non-aqueous reactive solutions like kerosene medium are valid cases for applying the equilibrium constant expression.
Final Answer: D. Solution of kerosene. -
Question 48 of 100
48. Question
1 pointsIf molar concentration of reactant “A” = 1, then rate of reaction = ?
Correct
Explanation: C
Step-by-Step Explanation:
1. General rate law:
Rate = k[A]n
where:
k = rate constant,
[A] = molar concentration of reactant A,
n = order of reaction w.r.t. A.
2. Given condition:
[A]=1 M
So,
Rate = k(1)n
3. Interpretation:
No matter what the order (n) is, raising 1 to any power still gives 1.
Therefore, the rate of reaction equals the rate constant.
Why other options are wrong:
A. Greater than k: Only possible if [A] > 1, not here.
B. Less than k: Only possible if [A] < 1, not here.
D. None: Incorrect since a clear answer exists.
Final Answer: C. Equal to k.Incorrect
Explanation: C
Step-by-Step Explanation:
1. General rate law:
Rate = k[A]n
where:
k = rate constant,
[A] = molar concentration of reactant A,
n = order of reaction w.r.t. A.
2. Given condition:
[A]=1 M
So,
Rate = k(1)n
3. Interpretation:
No matter what the order (n) is, raising 1 to any power still gives 1.
Therefore, the rate of reaction equals the rate constant.
Why other options are wrong:
A. Greater than k: Only possible if [A] > 1, not here.
B. Less than k: Only possible if [A] < 1, not here.
D. None: Incorrect since a clear answer exists.
Final Answer: C. Equal to k. -
Question 49 of 100
49. Question
1 pointsIn multi-step reactions, rate depends on:
Correct
Explanation: B
1. Multi-step reactions:
• Many reactions occur in a series of elementary steps.
• Each step can have a different speed (rate).
2. Rate-determining step (RDS):
• The slowest step controls the overall rate of the reaction.
• It acts like a “bottleneck” in a factory line → the entire process cannot go faster than this step.
3. Why other options are wrong:
• A. Fastest step: Has no control over overall rate because the slow step limits progress.
• C. Intermediate step: Intermediates are formed and consumed; their existence doesn’t set the overall rate directly.
• D. First step: Not always true — sometimes the first step is fast, and a later step is slow (that slow step still controls the rate).
Final Answer: B. Slowest step.Incorrect
Explanation: B
1. Multi-step reactions:
• Many reactions occur in a series of elementary steps.
• Each step can have a different speed (rate).
2. Rate-determining step (RDS):
• The slowest step controls the overall rate of the reaction.
• It acts like a “bottleneck” in a factory line → the entire process cannot go faster than this step.
3. Why other options are wrong:
• A. Fastest step: Has no control over overall rate because the slow step limits progress.
• C. Intermediate step: Intermediates are formed and consumed; their existence doesn’t set the overall rate directly.
• D. First step: Not always true — sometimes the first step is fast, and a later step is slow (that slow step still controls the rate).
Final Answer: B. Slowest step. -
Question 50 of 100
50. Question
1 pointsThe reaction whose rate doesn’t depend on concentration of reactants:
Correct
Explanation: B
1. Rate law general form:
Rate = k[A]n
n = 0 → zero-order reaction.
n = 1 → first-order reaction.
n = 2 → second-order reaction.
2. Zero-order reaction:
If n = 0:
Rate = k[A]0 = k
This means the rate is constant and does not depend on the concentration of reactant A.
Examples of zero-order reactions:
Photochemical reactions (e.g., decomposition of HI under light).
Catalytic reactions on metal surfaces (e.g., decomposition of NH3 on hot Pt).
Why other options are wrong:
A. Not possible: Incorrect — zero-order reactions are real and well-documented.
C. First order: Rate depends directly on concentration.
D. Second order: Rate depends on the square of concentration (or product of two concentrations).
Final Answer: B. Zero order.Incorrect
Explanation: B
1. Rate law general form:
Rate = k[A]n
n = 0 → zero-order reaction.
n = 1 → first-order reaction.
n = 2 → second-order reaction.
2. Zero-order reaction:
If n = 0:
Rate = k[A]0 = k
This means the rate is constant and does not depend on the concentration of reactant A.
Examples of zero-order reactions:
Photochemical reactions (e.g., decomposition of HI under light).
Catalytic reactions on metal surfaces (e.g., decomposition of NH3 on hot Pt).
Why other options are wrong:
A. Not possible: Incorrect — zero-order reactions are real and well-documented.
C. First order: Rate depends directly on concentration.
D. Second order: Rate depends on the square of concentration (or product of two concentrations).
Final Answer: B. Zero order. -
Question 51 of 100
51. Question
1 pointsIce can be melted at a temperature below 0°C by applying pressure. The process is an example of a:
Correct
Explanation: B
1. Process described:
• Ice (solid water) melts to form liquid water.
• This is a phase change, not the formation of a new substance.
2. Why pressure can melt ice below 0°C:
• According to the Clausius–Clapeyron relation, the melting point of ice decreases with increasing pressure.
• Pressure disrupts the open crystal structure of ice, making it easier for water molecules to rearrange into the denser liquid form.
• This is why ice skates glide — pressure of the blade lowers the melting point, forming a thin layer of water.
2. Classification of the change:
• It is physical because the chemical composition (H2O) remains the same.
• It is not chemical, since no new substance forms.
• It is not always spontaneous — external pressure must be applied.
Why other options are wrong:
• B. Non-spontaneous physical change: Not correct, because once pressure is applied, the change occurs naturally (no continuous external energy other than pressure).
• C. Spontaneous physical change: Not strictly correct — at temperatures below 0°C, ice will not melt spontaneously without applied pressure.
• D. Non-spontaneous chemical change: Wrong, because it is not chemical at all.
Final Answer: A. Physical change.Incorrect
Explanation: B
1. Process described:
• Ice (solid water) melts to form liquid water.
• This is a phase change, not the formation of a new substance.
2. Why pressure can melt ice below 0°C:
• According to the Clausius–Clapeyron relation, the melting point of ice decreases with increasing pressure.
• Pressure disrupts the open crystal structure of ice, making it easier for water molecules to rearrange into the denser liquid form.
• This is why ice skates glide — pressure of the blade lowers the melting point, forming a thin layer of water.
2. Classification of the change:
• It is physical because the chemical composition (H2O) remains the same.
• It is not chemical, since no new substance forms.
• It is not always spontaneous — external pressure must be applied.
Why other options are wrong:
• B. Non-spontaneous physical change: Not correct, because once pressure is applied, the change occurs naturally (no continuous external energy other than pressure).
• C. Spontaneous physical change: Not strictly correct — at temperatures below 0°C, ice will not melt spontaneously without applied pressure.
• D. Non-spontaneous chemical change: Wrong, because it is not chemical at all.
Final Answer: A. Physical change. -
Question 52 of 100
52. Question
1 pointsThe amount of heat evolved or absorbed in a chemical reaction is the same whether the reaction occurs in one step or in several steps. This is the statement of:
Correct
Explanation: B
1. Hess’s Law of Constant Heat Summation:
States that:
“The total enthalpy change (ΔH) of a reaction is the same whether the reaction occurs in a single step or through multiple steps.”
Mathematically:
ΔHoverall = ∑ΔHindividual steps
2. Reason:
• Enthalpy is a state function (depends only on initial and final states, not the path).
• Therefore, heat evolved/absorbed is independent of the pathway.
3. Why other options are wrong:
• A. First law of thermodynamics: Deals with conservation of energy, but not specifically about multi-step vs single-step reactions.
• C. Law of mass action: Relates reaction rate to concentrations, not heat changes.
• D. Raoult’s law: Deals with vapor pressure of solutions, not enthalpy.
Final Answer: B. Hess’s law.Incorrect
Explanation: B
1. Hess’s Law of Constant Heat Summation:
States that:
“The total enthalpy change (ΔH) of a reaction is the same whether the reaction occurs in a single step or through multiple steps.”
Mathematically:
ΔHoverall = ∑ΔHindividual steps
2. Reason:
• Enthalpy is a state function (depends only on initial and final states, not the path).
• Therefore, heat evolved/absorbed is independent of the pathway.
3. Why other options are wrong:
• A. First law of thermodynamics: Deals with conservation of energy, but not specifically about multi-step vs single-step reactions.
• C. Law of mass action: Relates reaction rate to concentrations, not heat changes.
• D. Raoult’s law: Deals with vapor pressure of solutions, not enthalpy.
Final Answer: B. Hess’s law. -
Question 53 of 100
53. Question
1 pointsOxidation is a chemical process in which a substance:
Correct
Explanation: D
Oxidation can be defined in different ways (all equivalent in chemistry):
1. Oxygen definition (classical):
Oxidation = gain of oxygen.
Example:
2Mg + O2 → 2MgO
Magnesium is oxidized (gains oxygen).
2. Hydrogen definition:
Oxidation = loss of hydrogen.
Example:
H2S → S+2H+
H2S is oxidized (loses hydrogen).
3. Electron definition (modern/Redox):
Oxidation = loss of electrons.
Example:
Na → Na+ + e−
Sodium is oxidized (loses an electron).
Why D is correct:
Since oxidation can mean gain of O, loss of H, or loss of e⁻, all three statements (A, B, C) are valid.
Final Answer: D. All of the above.Incorrect
Explanation: D
Oxidation can be defined in different ways (all equivalent in chemistry):
1. Oxygen definition (classical):
Oxidation = gain of oxygen.
Example:
2Mg + O2 → 2MgO
Magnesium is oxidized (gains oxygen).
2. Hydrogen definition:
Oxidation = loss of hydrogen.
Example:
H2S → S+2H+
H2S is oxidized (loses hydrogen).
3. Electron definition (modern/Redox):
Oxidation = loss of electrons.
Example:
Na → Na+ + e−
Sodium is oxidized (loses an electron).
Why D is correct:
Since oxidation can mean gain of O, loss of H, or loss of e⁻, all three statements (A, B, C) are valid.
Final Answer: D. All of the above. -
Question 54 of 100
54. Question
1 pointsOxidation state of manganese in KMnO4 is
Correct
Explanation: B
1. Given compound: Potassium permanganate → KMnO4.
2. Oxidation state rules applied:
• Potassium (K) is an alkali metal → always +1.
• Oxygen (O) is usually −2. With 4 oxygens: 4 ×−2 = −8.
• Let the oxidation state of manganese = xxx.
Equation:
(+1) + (x) + (−8) = 0
x – 7 = 0
x = +7
3. Result:
• Oxidation state of Mn in KMnO4 is +7.
Why other options are wrong:
A. +5, C. +3, D. +2: Possible oxidation states of manganese in other compounds (e.g., MnO2 = +4, MnCl2, Mn2O3 = +3), but not in KMnO4.
Final Answer: B. +7.Incorrect
Explanation: B
1. Given compound: Potassium permanganate → KMnO4.
2. Oxidation state rules applied:
• Potassium (K) is an alkali metal → always +1.
• Oxygen (O) is usually −2. With 4 oxygens: 4 ×−2 = −8.
• Let the oxidation state of manganese = xxx.
Equation:
(+1) + (x) + (−8) = 0
x – 7 = 0
x = +7
3. Result:
• Oxidation state of Mn in KMnO4 is +7.
Why other options are wrong:
A. +5, C. +3, D. +2: Possible oxidation states of manganese in other compounds (e.g., MnO2 = +4, MnCl2, Mn2O3 = +3), but not in KMnO4.
Final Answer: B. +7. -
Question 55 of 100
55. Question
1 pointsPropanoyl chloride is a member of:
Correct
Explanation: D
Propanoyl chloride has the structure CH3–CH2–CO–Cl. The –CO–Cl unit is an acyl (acid) halide functional group with general formula R–CO–X (X = Cl, Br). It is derived from the carboxylic acid propanoic acid (CH₃–CH₂–COOH) by replacing the –OH with Cl, so it’s classified as an acid (acyl) halide and named propanoyl chloride (common name: propionyl chloride).
Why not the others?
A. Alkyl halides: These are R–X (e.g., CH3–CH2–CH2–Cl) with no carbonyl. Propanoyl chloride includes a carbonyl (C=O), so it’s not an alkyl halide.
B. Acid amides: Amides are R–CO–NH₂ / –NHR / –NR₂ (e.g., propanamide CH3–CH2–CONH2). There’s no –NH group in propanoyl chloride.
C. Acid anhydrides: Anhydrides are R–CO–O–CO–R′ (e.g., (CH3–CH2–CO)2O). Propanoyl chloride lacks the –CO–O–CO– linkage.
Extra exam-tip:
Acid halides readily undergo nucleophilic acyl substitution (e.g., to amides, esters, acids, or anhydrides), whereas alkyl halides typically undergo SN1/SN2 reactions—another quick way to tell the classes apart.Incorrect
Explanation: D
Propanoyl chloride has the structure CH3–CH2–CO–Cl. The –CO–Cl unit is an acyl (acid) halide functional group with general formula R–CO–X (X = Cl, Br). It is derived from the carboxylic acid propanoic acid (CH₃–CH₂–COOH) by replacing the –OH with Cl, so it’s classified as an acid (acyl) halide and named propanoyl chloride (common name: propionyl chloride).
Why not the others?
A. Alkyl halides: These are R–X (e.g., CH3–CH2–CH2–Cl) with no carbonyl. Propanoyl chloride includes a carbonyl (C=O), so it’s not an alkyl halide.
B. Acid amides: Amides are R–CO–NH₂ / –NHR / –NR₂ (e.g., propanamide CH3–CH2–CONH2). There’s no –NH group in propanoyl chloride.
C. Acid anhydrides: Anhydrides are R–CO–O–CO–R′ (e.g., (CH3–CH2–CO)2O). Propanoyl chloride lacks the –CO–O–CO– linkage.
Extra exam-tip:
Acid halides readily undergo nucleophilic acyl substitution (e.g., to amides, esters, acids, or anhydrides), whereas alkyl halides typically undergo SN1/SN2 reactions—another quick way to tell the classes apart. -
Question 56 of 100
56. Question
1 pointsWhat are the indirect effects of a rise in heart rate for an athlete during a 200-meter race?
Correct
Explanation: C
1. During a sprint (200 m race):
The muscles require rapid delivery of oxygen and nutrients (especially glucose).
The heart rate increases → more blood is pumped per minute → more oxygen and glucose are delivered to muscle cells.
Waste products (like CO2 and lactic acid) are removed faster.
2. Looking at options:
A. Increase digestion of carbohydrates → Not correct. Digestion is slowed during exercise because blood is diverted away from the gut to the muscles.
B. Increase ventilation of the lungs with fresh air → This is mainly due to increased breathing rate, not directly an effect of increased heart rate (though both are coordinated). Heart rate rise itself doesn’t increase ventilation.
C. Reduce the need for anaerobic respiration Correct. By pumping more oxygen-rich blood to the muscles, the increased heart rate helps supply sufficient oxygen, thereby delaying or reducing the need for anaerobic respiration (which produces lactic acid).
D. Reduce the need for sweating to cool the body → Incorrect. Sweating is mainly controlled by body temperature, not heart rate.
Correct Answer: C. To reduce the need for anaerobic respiration:
The indirect effect of increased heart rate is enhanced oxygen delivery to muscles. This allows muscles to rely more on aerobic respiration, which is efficient and prevents early buildup of lactic acid, thus reducing the reliance on anaerobic respiration.Incorrect
Explanation: C
1. During a sprint (200 m race):
The muscles require rapid delivery of oxygen and nutrients (especially glucose).
The heart rate increases → more blood is pumped per minute → more oxygen and glucose are delivered to muscle cells.
Waste products (like CO2 and lactic acid) are removed faster.
2. Looking at options:
A. Increase digestion of carbohydrates → Not correct. Digestion is slowed during exercise because blood is diverted away from the gut to the muscles.
B. Increase ventilation of the lungs with fresh air → This is mainly due to increased breathing rate, not directly an effect of increased heart rate (though both are coordinated). Heart rate rise itself doesn’t increase ventilation.
C. Reduce the need for anaerobic respiration Correct. By pumping more oxygen-rich blood to the muscles, the increased heart rate helps supply sufficient oxygen, thereby delaying or reducing the need for anaerobic respiration (which produces lactic acid).
D. Reduce the need for sweating to cool the body → Incorrect. Sweating is mainly controlled by body temperature, not heart rate.
Correct Answer: C. To reduce the need for anaerobic respiration:
The indirect effect of increased heart rate is enhanced oxygen delivery to muscles. This allows muscles to rely more on aerobic respiration, which is efficient and prevents early buildup of lactic acid, thus reducing the reliance on anaerobic respiration. -
Question 57 of 100
57. Question
1 pointsBlood from the ileum is carried in the hepatic portal vein to the liver. Why is this an advantage to the body?
Correct
Explanation: B
1. Blood from the ileum contains digested nutrients (glucose, amino acids, some vitamins, toxins if present, etc.).
• These nutrients go via the hepatic portal vein directly to the liver before entering general circulation.
2. Checking options one by one:
A. Amino acids → urea before circulation
The liver deaminates excess amino acids, but not immediately all amino acids. Useful ones are kept for protein synthesis. Urea production happens when there is surplus amino acid metabolism, not the main advantage.
B. Excess glucose → glycogen
The liver stores glucose as glycogen (glycogenesis). This prevents large fluctuations of blood glucose and avoids wastage. This is a major advantage of portal blood going through the liver.
C. Fat products
Fats are absorbed into lacteals (lymphatic system), not directly into the hepatic portal vein, so this option is wrong.
D. Toxins destroyed before reaching body cells (also true!)
The liver detoxifies substances (e.g., alcohol, drugs, poisons). This is another major advantage of hepatic portal circulation.
Correct Answer: B. Excess glucose can be converted to glycogen for storage and not excreted
Why not D?
Option D is also partly correct because the liver detoxifies harmful substances, but the classic, expected textbook answer for this question (especially at
FSc/entry test level) is B, since it directly highlights the role of the liver in glucose homeostasis after absorption from the ileum.
Final Key: B
Explanation: The hepatic portal vein ensures that absorbed glucose from the ileum is first regulated by the liver. Excess glucose is stored as glycogen, preventing it from being lost or causing harmful spikes in blood sugar levels.Incorrect
Explanation: B
1. Blood from the ileum contains digested nutrients (glucose, amino acids, some vitamins, toxins if present, etc.).
• These nutrients go via the hepatic portal vein directly to the liver before entering general circulation.
2. Checking options one by one:
A. Amino acids → urea before circulation
The liver deaminates excess amino acids, but not immediately all amino acids. Useful ones are kept for protein synthesis. Urea production happens when there is surplus amino acid metabolism, not the main advantage.
B. Excess glucose → glycogen
The liver stores glucose as glycogen (glycogenesis). This prevents large fluctuations of blood glucose and avoids wastage. This is a major advantage of portal blood going through the liver.
C. Fat products
Fats are absorbed into lacteals (lymphatic system), not directly into the hepatic portal vein, so this option is wrong.
D. Toxins destroyed before reaching body cells (also true!)
The liver detoxifies substances (e.g., alcohol, drugs, poisons). This is another major advantage of hepatic portal circulation.
Correct Answer: B. Excess glucose can be converted to glycogen for storage and not excreted
Why not D?
Option D is also partly correct because the liver detoxifies harmful substances, but the classic, expected textbook answer for this question (especially at
FSc/entry test level) is B, since it directly highlights the role of the liver in glucose homeostasis after absorption from the ileum.
Final Key: B
Explanation: The hepatic portal vein ensures that absorbed glucose from the ileum is first regulated by the liver. Excess glucose is stored as glycogen, preventing it from being lost or causing harmful spikes in blood sugar levels. -
Question 58 of 100
58. Question
1 pointsAfter eating, pH of the mouth decreases. Which statement explains this?
Correct
Explanation: A
1. What happens after eating?
Food (especially sugars and starches) sticks to teeth and remains in the mouth.
Bacteria in dental plaque ferment these sugars and release organic acids (like lactic acid).
These acids lower the pH of the mouth, making it more acidic.
2. Check the options:
A. Bacteria release acids (Correct). This is the true cause of decreased pH.
B. Enzymes in saliva release acids Salivary enzymes (like amylase) break starch into maltose but do not release acids.
C. Salivary glands release acids Saliva is actually slightly alkaline, containing bicarbonates that neutralize acids, not produce them.
D. Sensory neurons in tongue release acids Neurons do not release acids; they only detect taste stimuli.
Correct Answer: A. Bacteria release acids
After eating, especially sugary foods, oral bacteria metabolize sugars and produce acids as waste products. These acids decrease mouth pH, which can eventually lead to tooth enamel erosion and cavities.Incorrect
Explanation: A
1. What happens after eating?
Food (especially sugars and starches) sticks to teeth and remains in the mouth.
Bacteria in dental plaque ferment these sugars and release organic acids (like lactic acid).
These acids lower the pH of the mouth, making it more acidic.
2. Check the options:
A. Bacteria release acids (Correct). This is the true cause of decreased pH.
B. Enzymes in saliva release acids Salivary enzymes (like amylase) break starch into maltose but do not release acids.
C. Salivary glands release acids Saliva is actually slightly alkaline, containing bicarbonates that neutralize acids, not produce them.
D. Sensory neurons in tongue release acids Neurons do not release acids; they only detect taste stimuli.
Correct Answer: A. Bacteria release acids
After eating, especially sugary foods, oral bacteria metabolize sugars and produce acids as waste products. These acids decrease mouth pH, which can eventually lead to tooth enamel erosion and cavities. -
Question 59 of 100
59. Question
1 pointsWhich statement about the Ulna is correct?
Correct
Explanation: B
A. Lateral bone, thumb side (Wrong) → that’s the radius. The ulna lies on the little finger side (medial side).
B. Proximal end forms elbow joint with humerus (Correct) → the trochlear notch of ulna articulates with the trochlea of humerus, and the olecranon process forms the elbow tip.
C. Directly articulates with carpals (Wrong) → only the radius articulates with carpals; the ulna does not.
D. No role in elbow joint (Wrong) → it plays the main role in the elbow joint.
Correct Answer: B. Its proximal end forms the elbow joint with the humerus
The ulna is the medial bone of the forearm. At its proximal end, the olecranon process (elbow tip) and trochlear notch fit with the humerus, forming the hinge joint of the elbow. Distally, it does not directly articulate with the wrist — the radius does that.Incorrect
Explanation: B
A. Lateral bone, thumb side (Wrong) → that’s the radius. The ulna lies on the little finger side (medial side).
B. Proximal end forms elbow joint with humerus (Correct) → the trochlear notch of ulna articulates with the trochlea of humerus, and the olecranon process forms the elbow tip.
C. Directly articulates with carpals (Wrong) → only the radius articulates with carpals; the ulna does not.
D. No role in elbow joint (Wrong) → it plays the main role in the elbow joint.
Correct Answer: B. Its proximal end forms the elbow joint with the humerus
The ulna is the medial bone of the forearm. At its proximal end, the olecranon process (elbow tip) and trochlear notch fit with the humerus, forming the hinge joint of the elbow. Distally, it does not directly articulate with the wrist — the radius does that. -
Question 60 of 100
60. Question
1 pointsTwo brothers: one blood group A, one blood group O. What about parents?
Correct
Explanation: A
ABO alleles: IA,IB,i.
IA and IB are dominant over ii; IA & IB are codominant.
Phenotypes & genotypes:
Group A = IAIA or IA i
Group O = ii (must receive ii from both parents)
Since one brother is O (ii), each parent must contribute an ii allele.
Since the other brother is A, at least one parent must carry an IAIA allele. Combining these facts:
Possible parent genotype pairs that can produce A and O siblings:
1. IAi × IAi → children: A (AA/Ai) and O (ii)
2. IAi × ii → children: A (Ai) and O (ii)
3. IAi × IBi → children: A, B, AB, O (so A and O possible)
In every valid case, at least one parent is heterozygous (carries two different alleles).
Why not the others?
B. Both parents must be heterozygous Not necessary (e.g., IAi × ii works).
C. Both parents homozygous Impossible to get both A and O siblings from two homozygous parents.
D. One parent (at least) homozygous Not required (e.g., IAi × IAi both heterozygous).
Bottom line: To have an O child, both parents must carry ii; to also have an A child, someone must carry IA. The statement that always holds among the options is A.Incorrect
Explanation: A
ABO alleles: IA,IB,i.
IA and IB are dominant over ii; IA & IB are codominant.
Phenotypes & genotypes:
Group A = IAIA or IA i
Group O = ii (must receive ii from both parents)
Since one brother is O (ii), each parent must contribute an ii allele.
Since the other brother is A, at least one parent must carry an IAIA allele. Combining these facts:
Possible parent genotype pairs that can produce A and O siblings:
1. IAi × IAi → children: A (AA/Ai) and O (ii)
2. IAi × ii → children: A (Ai) and O (ii)
3. IAi × IBi → children: A, B, AB, O (so A and O possible)
In every valid case, at least one parent is heterozygous (carries two different alleles).
Why not the others?
B. Both parents must be heterozygous Not necessary (e.g., IAi × ii works).
C. Both parents homozygous Impossible to get both A and O siblings from two homozygous parents.
D. One parent (at least) homozygous Not required (e.g., IAi × IAi both heterozygous).
Bottom line: To have an O child, both parents must carry ii; to also have an A child, someone must carry IA. The statement that always holds among the options is A. -
Question 61 of 100
61. Question
1 pointsDamage to the cerebellum causes:
Correct
Explanation: A
1. Cerebellum function:
Located at the back of the brain, below the cerebrum.
Main role: control and coordination of voluntary movements, balance, posture, and fine motor skills.
It does not initiate movements but makes them smooth and coordinated.
2. Check options:
A. Poor coordination of movements (Correct) → Damage leads to ataxia (loss of coordination, unsteady gait, tremors).
B. Lack of temperature control Controlled by the hypothalamus, not cerebellum.
C. Irregular heartbeat Controlled by the medulla oblongata (cardiac center), not cerebellum.
D. Inability to recognize visual stimuli That’s a problem of the occipital lobe of cerebrum, not cerebellum.
Correct Answer: A. Poor coordination of movements
The cerebellum ensures smooth, balanced, and precise muscle activity. Damage causes ataxia, tremors, and difficulty in coordinated tasks (e.g., walking straight, touching nose with finger).Incorrect
Explanation: A
1. Cerebellum function:
Located at the back of the brain, below the cerebrum.
Main role: control and coordination of voluntary movements, balance, posture, and fine motor skills.
It does not initiate movements but makes them smooth and coordinated.
2. Check options:
A. Poor coordination of movements (Correct) → Damage leads to ataxia (loss of coordination, unsteady gait, tremors).
B. Lack of temperature control Controlled by the hypothalamus, not cerebellum.
C. Irregular heartbeat Controlled by the medulla oblongata (cardiac center), not cerebellum.
D. Inability to recognize visual stimuli That’s a problem of the occipital lobe of cerebrum, not cerebellum.
Correct Answer: A. Poor coordination of movements
The cerebellum ensures smooth, balanced, and precise muscle activity. Damage causes ataxia, tremors, and difficulty in coordinated tasks (e.g., walking straight, touching nose with finger). -
Question 62 of 100
62. Question
1 pointsWhich is the drinking process of the cell?
Correct
Explanation: C
1. Phagocytosis = “cell eating” → intake of solid particles (like bacteria or food particles).
2. Pinocytosis = “cell drinking” → intake of extracellular fluid and dissolved substances into small vesicles.
3. Exocytosis = expulsion/secretion of materials out of the cell (reverse of endocytosis).
4. Chloroplast = organelle for photosynthesis in plants, not a process.
Correct Answer: C. Pinocytosis
Pinocytosis is a type of endocytosis where the cell engulfs droplets of extracellular fluid. It’s called the “cell drinking” process, as opposed to phagocytosis (cell eating).Incorrect
Explanation: C
1. Phagocytosis = “cell eating” → intake of solid particles (like bacteria or food particles).
2. Pinocytosis = “cell drinking” → intake of extracellular fluid and dissolved substances into small vesicles.
3. Exocytosis = expulsion/secretion of materials out of the cell (reverse of endocytosis).
4. Chloroplast = organelle for photosynthesis in plants, not a process.
Correct Answer: C. Pinocytosis
Pinocytosis is a type of endocytosis where the cell engulfs droplets of extracellular fluid. It’s called the “cell drinking” process, as opposed to phagocytosis (cell eating). -
Question 63 of 100
63. Question
1 pointsSynthesis of glycogen is known as:
Correct
Explanation: D
1. Gluconeogenesis = formation of glucose from non-carbohydrate sources (like amino acids, lactate, glycerol).
2. Glycogenolysis = breakdown of glycogen into glucose (for energy release).
3. Glycolysis = breakdown of glucose to pyruvate with ATP release (first step of respiration).
4. Glycogenesis = synthesis of glycogen from glucose molecules for storage in liver and muscle.
Correct Answer: D. Glycogenesis
When blood glucose levels are high (after a meal), glucose is converted into glycogen in the liver and muscle cells through glycogenesis. This process is stimulated by insulin.Incorrect
Explanation: D
1. Gluconeogenesis = formation of glucose from non-carbohydrate sources (like amino acids, lactate, glycerol).
2. Glycogenolysis = breakdown of glycogen into glucose (for energy release).
3. Glycolysis = breakdown of glucose to pyruvate with ATP release (first step of respiration).
4. Glycogenesis = synthesis of glycogen from glucose molecules for storage in liver and muscle.
Correct Answer: D. Glycogenesis
When blood glucose levels are high (after a meal), glucose is converted into glycogen in the liver and muscle cells through glycogenesis. This process is stimulated by insulin. -
Question 64 of 100
64. Question
1 pointsAll are disaccharides except:
Correct
Explanation: D
1. Maltose = Glucose + Glucose → disaccharide
2. Sucrose = Glucose + Fructose → disaccharide
3. Lactose = Glucose + Galactose → disaccharide
4. Fructose = a monosaccharide (single sugar, along with glucose and galactose)
Correct Answer: D. Fructose
Disaccharides are formed by the condensation of two monosaccharides. Maltose, sucrose, and lactose all belong to disaccharides, while fructose is a monosaccharide (a simple sugar).Incorrect
Explanation: D
1. Maltose = Glucose + Glucose → disaccharide
2. Sucrose = Glucose + Fructose → disaccharide
3. Lactose = Glucose + Galactose → disaccharide
4. Fructose = a monosaccharide (single sugar, along with glucose and galactose)
Correct Answer: D. Fructose
Disaccharides are formed by the condensation of two monosaccharides. Maltose, sucrose, and lactose all belong to disaccharides, while fructose is a monosaccharide (a simple sugar). -
Question 65 of 100
65. Question
1 pointsCovalent bond between glucose and fructose is called:
Correct
Explanation: A
1. Glucose + Fructose → Sucrose (a disaccharide).
The covalent linkage formed between the anomeric carbon of glucose and fructose is a glycosidic bond (specifically, an α-1,2-glycosidic bond).
2. Peptide bond → links amino acids in proteins
3. Ester bond → links fatty acids to glycerol in lipids
4. Hydrogen bond → weak attraction between molecules, not the covalent linkage here
Correct Answer: A. Glycosidic bond
In disaccharides like sucrose, monosaccharides are joined by a glycosidic linkage. In sucrose, it is specifically an α-1,2-glycosidic bond between glucose and fructose.Incorrect
Explanation: A
1. Glucose + Fructose → Sucrose (a disaccharide).
The covalent linkage formed between the anomeric carbon of glucose and fructose is a glycosidic bond (specifically, an α-1,2-glycosidic bond).
2. Peptide bond → links amino acids in proteins
3. Ester bond → links fatty acids to glycerol in lipids
4. Hydrogen bond → weak attraction between molecules, not the covalent linkage here
Correct Answer: A. Glycosidic bond
In disaccharides like sucrose, monosaccharides are joined by a glycosidic linkage. In sucrose, it is specifically an α-1,2-glycosidic bond between glucose and fructose. -
Question 66 of 100
66. Question
1 pointsAn enzyme without its cofactor is called:
Correct
Explanation: D
1. Enzyme structure:
• Many enzymes need a cofactor (non-protein helper, e.g., metal ion or organic molecule) to work.
• The complete, active form of enzyme = Holoenzyme = Apoenzyme + Cofactor.
2. Definitions:
• Active site = region on enzyme where substrate binds
• Coenzyme = organic, non-protein cofactor (like NAD+, FAD)
• Prosthetic group = tightly bound cofactor to enzyme
• Apoenzyme = the protein part of enzyme, inactive without cofactor
Correct Answer: D. Apoenzyme
An apoenzyme is the inactive protein part of an enzyme. When the required cofactor (metal ion, coenzyme, or prosthetic group) binds, it becomes a holoenzyme, which is catalytically active.Incorrect
Explanation: D
1. Enzyme structure:
• Many enzymes need a cofactor (non-protein helper, e.g., metal ion or organic molecule) to work.
• The complete, active form of enzyme = Holoenzyme = Apoenzyme + Cofactor.
2. Definitions:
• Active site = region on enzyme where substrate binds
• Coenzyme = organic, non-protein cofactor (like NAD+, FAD)
• Prosthetic group = tightly bound cofactor to enzyme
• Apoenzyme = the protein part of enzyme, inactive without cofactor
Correct Answer: D. Apoenzyme
An apoenzyme is the inactive protein part of an enzyme. When the required cofactor (metal ion, coenzyme, or prosthetic group) binds, it becomes a holoenzyme, which is catalytically active. -
Question 67 of 100
67. Question
1 pointsCoenzymes are primarily derived from:
Correct
Explanation: A
Coenzymes are organic cofactors; many arise from B‑complex vitamins (e.g., NAD⁺ from niacin).Incorrect
Explanation: A
Coenzymes are organic cofactors; many arise from B‑complex vitamins (e.g., NAD⁺ from niacin). -
Question 68 of 100
68. Question
1 pointsWhich statement correctly describes the nucleotide triphosphates used in nucleic‑acid synthesis?
Correct
Explanation: A
RNA polymerases incorporate ATP/GTP/CTP/UTP; DNA polymerases incorporate the deoxy forms.Incorrect
Explanation: A
RNA polymerases incorporate ATP/GTP/CTP/UTP; DNA polymerases incorporate the deoxy forms. -
Question 69 of 100
69. Question
1 pointsThe first stable product of the dark reaction is a _____________ carbon molecule:
Correct
Explanation: C
1. The dark reaction (Calvin cycle) occurs in the stroma of chloroplasts.
2. CO2 is fixed by the enzyme RuBisCO, combining CO2 with RuBP (5C compound) to form a short-lived 6C compound.
3. This unstable 6C compound immediately splits into two molecules of 3-phosphoglycerate (3-PGA).
4. 3-PGA (3-phosphoglycerate) is the first stable product of the Calvin cycle.
Correct Answer: C. 3
Although a 6-carbon intermediate forms first, it is unstable and immediately breaks down. The first stable compound is 3-phosphoglycerate (3C), which is why the Calvin cycle is also called the C3 pathway.Incorrect
Explanation: C
1. The dark reaction (Calvin cycle) occurs in the stroma of chloroplasts.
2. CO2 is fixed by the enzyme RuBisCO, combining CO2 with RuBP (5C compound) to form a short-lived 6C compound.
3. This unstable 6C compound immediately splits into two molecules of 3-phosphoglycerate (3-PGA).
4. 3-PGA (3-phosphoglycerate) is the first stable product of the Calvin cycle.
Correct Answer: C. 3
Although a 6-carbon intermediate forms first, it is unstable and immediately breaks down. The first stable compound is 3-phosphoglycerate (3C), which is why the Calvin cycle is also called the C3 pathway. -
Question 70 of 100
70. Question
1 pointsTobacco mosaic virus is:
Correct
Explanation: C
1. Tobacco mosaic virus (TMV):
• It was the first virus discovered (by Ivanovsky, later studied by Beijerinck).
• Structure: rod-shaped, rigid, and helical.
• Made of a single-stranded RNA genome surrounded by protein subunits arranged helically.
2. Check options:
A. Spherical That describes viruses like polio virus.
B. Tadpole-like That’s typical of bacteriophages (head + tail).
C. Rod-shaped Correct, TMV is rod-shaped.
D. Polyhedral Example: Adenovirus.
Correct Answer: C. Rod-shaped
TMV is a rigid rod-shaped plant virus with helical symmetry. Its discovery was key in understanding that viruses are non-cellular infectious agents made of nucleic acid and protein.Incorrect
Explanation: C
1. Tobacco mosaic virus (TMV):
• It was the first virus discovered (by Ivanovsky, later studied by Beijerinck).
• Structure: rod-shaped, rigid, and helical.
• Made of a single-stranded RNA genome surrounded by protein subunits arranged helically.
2. Check options:
A. Spherical That describes viruses like polio virus.
B. Tadpole-like That’s typical of bacteriophages (head + tail).
C. Rod-shaped Correct, TMV is rod-shaped.
D. Polyhedral Example: Adenovirus.
Correct Answer: C. Rod-shaped
TMV is a rigid rod-shaped plant virus with helical symmetry. Its discovery was key in understanding that viruses are non-cellular infectious agents made of nucleic acid and protein. -
Question 71 of 100
71. Question
1 pointsAll of the following are viruses except:
Correct
Explanation: D
1. SARS = Severe Acute Respiratory Syndrome, caused by a coronavirus (SARS-CoV) → virus.
2. FIV = Feline Immunodeficiency Virus (retrovirus, affects cats) → virus.
3. SIV = Simian Immunodeficiency Virus (retrovirus, affects monkeys/apes) → virus.
4. Mycoplasma = A bacterium, the smallest free-living, wall-less prokaryote. Causes diseases like pneumonia in humans. → not a virus.
Correct Answer: D. Mycoplasma
SARS, FIV, and SIV are viruses, whereas Mycoplasma is a bacterium, distinguished by having no cell wall and being the smallest living cell capable of independent growth.Incorrect
Explanation: D
1. SARS = Severe Acute Respiratory Syndrome, caused by a coronavirus (SARS-CoV) → virus.
2. FIV = Feline Immunodeficiency Virus (retrovirus, affects cats) → virus.
3. SIV = Simian Immunodeficiency Virus (retrovirus, affects monkeys/apes) → virus.
4. Mycoplasma = A bacterium, the smallest free-living, wall-less prokaryote. Causes diseases like pneumonia in humans. → not a virus.
Correct Answer: D. Mycoplasma
SARS, FIV, and SIV are viruses, whereas Mycoplasma is a bacterium, distinguished by having no cell wall and being the smallest living cell capable of independent growth. -
Question 72 of 100
72. Question
1 pointsWhich type of hepatitis is transmitted by fecal–oral route?
Correct
Explanation: A
1. Hepatitis A virus (HAV):
• Transmitted by fecal–oral route (contaminated food, water).
• Common in areas with poor sanitation.
2. Hepatitis B, C, D:
Spread mainly by blood, body fluids, sexual contact, contaminated needles, and from mother to child, not fecal–oral.
Correct Answer: A. Hepatitis A
Hepatitis A is an enteric virus. It does not cause chronic infection, but can cause acute jaundice. Hepatitis B, C, and D are blood-borne viruses.Incorrect
Explanation: A
1. Hepatitis A virus (HAV):
• Transmitted by fecal–oral route (contaminated food, water).
• Common in areas with poor sanitation.
2. Hepatitis B, C, D:
Spread mainly by blood, body fluids, sexual contact, contaminated needles, and from mother to child, not fecal–oral.
Correct Answer: A. Hepatitis A
Hepatitis A is an enteric virus. It does not cause chronic infection, but can cause acute jaundice. Hepatitis B, C, and D are blood-borne viruses. -
Question 73 of 100
73. Question
1 pointsWhich type of archaea dominantly occurs in the Dead Sea?
Correct
Explanation: B
1. Dead Sea environment:
• Extremely high salt concentration (~10 times more than oceans).
• Hot temperatures, but the most striking feature is its salinity.
2. Types of archaea:
• Thermophiles → thrive in very high temperatures (hot springs, hydrothermal vents).
• Halophiles → thrive in extremely salty environments (like Dead Sea, Great Salt Lake).
• Methanogens → anaerobic archaea that produce methane, usually found in swamps, intestines, sewage.
4. Correct match:
• The Dead Sea is dominated by extreme halophiles, not thermophiles or methanogens.
Correct Answer: B. Halophiles
The Dead Sea’s extremely salty conditions support the growth of halophilic archaea, which possess special adaptations (e.g., proteins and cell walls stable in high salt) to survive.Incorrect
Explanation: B
1. Dead Sea environment:
• Extremely high salt concentration (~10 times more than oceans).
• Hot temperatures, but the most striking feature is its salinity.
2. Types of archaea:
• Thermophiles → thrive in very high temperatures (hot springs, hydrothermal vents).
• Halophiles → thrive in extremely salty environments (like Dead Sea, Great Salt Lake).
• Methanogens → anaerobic archaea that produce methane, usually found in swamps, intestines, sewage.
4. Correct match:
• The Dead Sea is dominated by extreme halophiles, not thermophiles or methanogens.
Correct Answer: B. Halophiles
The Dead Sea’s extremely salty conditions support the growth of halophilic archaea, which possess special adaptations (e.g., proteins and cell walls stable in high salt) to survive. -
Question 74 of 100
74. Question
1 pointsWhich of the following causes tuberculosis and leprosy?
Correct
Explanation: B
1. Tuberculosis (TB): caused by Mycobacterium tuberculosis.
2. Leprosy (Hansen’s disease): caused by Mycobacterium leprae.
3. Check the options:
A. Streptomyces → These are actinobacteria that produce antibiotics, not TB/leprosy.
B. Actinomycetes (Mycobacterium) → Correct group. Mycobacterium is an actinomycete responsible for TB & leprosy.
C. Mycoplasma → Wall-less bacteria causing pneumonia, not TB or leprosy.
D. None of these → Incorrect because B is correct.
Correct Answer: B. Actinomycetes (Mycobacterium)
Tuberculosis and leprosy are caused by Mycobacterium species, which belong to the group of Actinomycetes (Gram-positive bacteria with high lipid content in their cell walls, especially mycolic acid).Incorrect
Explanation: B
1. Tuberculosis (TB): caused by Mycobacterium tuberculosis.
2. Leprosy (Hansen’s disease): caused by Mycobacterium leprae.
3. Check the options:
A. Streptomyces → These are actinobacteria that produce antibiotics, not TB/leprosy.
B. Actinomycetes (Mycobacterium) → Correct group. Mycobacterium is an actinomycete responsible for TB & leprosy.
C. Mycoplasma → Wall-less bacteria causing pneumonia, not TB or leprosy.
D. None of these → Incorrect because B is correct.
Correct Answer: B. Actinomycetes (Mycobacterium)
Tuberculosis and leprosy are caused by Mycobacterium species, which belong to the group of Actinomycetes (Gram-positive bacteria with high lipid content in their cell walls, especially mycolic acid). -
Question 75 of 100
75. Question
1 pointsAll are bacillus bacteria except:
Correct
Explanation: D
1. Bacillus = rod-shaped bacteria.
• Pseudomonas → Gram-negative rod-shaped (bacillus).
• Clostridium → Gram-positive rod-shaped, spore-forming.
• Salmonella → Gram-negative rod-shaped, enteric bacteria.
• Streptococcus → Gram-positive spherical (coccus), forms chains.
2. Therefore, Streptococcus is not bacillus, it is a coccus.
Correct Answer: D. Streptococcus
Bacillus bacteria are rod-shaped, while cocci are spherical. Pseudomonas, Clostridium, and Salmonella are bacilli, but Streptococcus is a coccus (chain of spheres).Incorrect
Explanation: D
1. Bacillus = rod-shaped bacteria.
• Pseudomonas → Gram-negative rod-shaped (bacillus).
• Clostridium → Gram-positive rod-shaped, spore-forming.
• Salmonella → Gram-negative rod-shaped, enteric bacteria.
• Streptococcus → Gram-positive spherical (coccus), forms chains.
2. Therefore, Streptococcus is not bacillus, it is a coccus.
Correct Answer: D. Streptococcus
Bacillus bacteria are rod-shaped, while cocci are spherical. Pseudomonas, Clostridium, and Salmonella are bacilli, but Streptococcus is a coccus (chain of spheres). -
Question 76 of 100
76. Question
1 pointsThe hard exoskeleton of reef‑building corals (phylum Cnidaria) is composed primarily of:
Correct
Explanation: B
Corals secrete CaCO₃ to form reefs; many other cnidarians lack hard skeletons.Incorrect
Explanation: B
Corals secrete CaCO₃ to form reefs; many other cnidarians lack hard skeletons. -
Question 77 of 100
77. Question
1 pointsWater enters the sponge through:
Correct
Explanation: B
1. Sponges (Porifera):
• They have a canal system for water circulation.
• Ostia = tiny pores on the sponge body through which water enters.
• Water passes into the spongocoel (central cavity).
• It leaves the sponge through the osculum (large opening at the top).
• Madreporite → found in echinoderms (starfish), not sponges.
2. Checking options:
• A. Osculum (exit opening).
• B. Ostia (entry pores).
• C. Madreporite (not in sponges).
• D. Spongocoel (central cavity, not the entry point).
Correct Answer: B. Ostia
Water carrying food and oxygen enters a sponge’s body through ostia, circulates in the spongocoel, and exits via the osculum.Incorrect
Explanation: B
1. Sponges (Porifera):
• They have a canal system for water circulation.
• Ostia = tiny pores on the sponge body through which water enters.
• Water passes into the spongocoel (central cavity).
• It leaves the sponge through the osculum (large opening at the top).
• Madreporite → found in echinoderms (starfish), not sponges.
2. Checking options:
• A. Osculum (exit opening).
• B. Ostia (entry pores).
• C. Madreporite (not in sponges).
• D. Spongocoel (central cavity, not the entry point).
Correct Answer: B. Ostia
Water carrying food and oxygen enters a sponge’s body through ostia, circulates in the spongocoel, and exits via the osculum. -
Question 78 of 100
78. Question
1 pointsThe respiratory system gives the basic ability to:
A. Smell
B. Breathe
C. Give out CO2
D. None of theseCorrect
Explanation: B
1. Main function of the respiratory system:
• Exchange of gases → oxygen in, carbon dioxide out.
• This process is fundamentally breathing (ventilation + gas exchange).
2. Check options:
• A. Smell → Olfactory function of the nose, not the basic ability of the respiratory system.
• B. Breathe → This is the most direct, fundamental ability provided by the respiratory system.
• C. Give out CO2 → Correct but secondary; it is part of breathing.
Correct Answer: B. Breathe
The respiratory system’s basic ability is to breathe, which includes both oxygen intake and carbon dioxide removal. Smelling is an associated function, and CO2 exhalation is part of the process, but the primary ability is breathing.Incorrect
Explanation: B
1. Main function of the respiratory system:
• Exchange of gases → oxygen in, carbon dioxide out.
• This process is fundamentally breathing (ventilation + gas exchange).
2. Check options:
• A. Smell → Olfactory function of the nose, not the basic ability of the respiratory system.
• B. Breathe → This is the most direct, fundamental ability provided by the respiratory system.
• C. Give out CO2 → Correct but secondary; it is part of breathing.
Correct Answer: B. Breathe
The respiratory system’s basic ability is to breathe, which includes both oxygen intake and carbon dioxide removal. Smelling is an associated function, and CO2 exhalation is part of the process, but the primary ability is breathing. -
Question 79 of 100
79. Question
1 pointsA respiratory surface must be permeable, thin, have large surface area, and good supply of:
Correct
Explanation: C
A. Gases diffuse, but the surface itself needs blood supply to carry them.
B. Lymph Lymphatic system doesn’t transport respiratory gases.
C. Blood Correct → Blood continuously transports O₂ and CO₂, maintaining diffusion gradient.
D. None of these Wrong, because C is correct.
Correct Answer: C. BloodIncorrect
Explanation: C
A. Gases diffuse, but the surface itself needs blood supply to carry them.
B. Lymph Lymphatic system doesn’t transport respiratory gases.
C. Blood Correct → Blood continuously transports O₂ and CO₂, maintaining diffusion gradient.
D. None of these Wrong, because C is correct.
Correct Answer: C. Blood -
Question 80 of 100
80. Question
1 pointsThe bronchioles are located at the end of bronchi and terminate in:
Correct
Explanation: D
1. Pathway of air in humans:
Trachea → Primary bronchi → Secondary bronchi → Tertiary bronchi → Bronchioles → Alveolar ducts → Alveolar sacs → Alveoli.
2. Alveoli are the tiny air sacs where gas exchange occurs.
3. Alveolar sacs are clusters (bunches) of alveoli at the ends of bronchioles.
4. So, bronchioles don’t end directly in a single alveolus but in alveolar sacs made up of many alveoli.
Correct Answer: D. Both B and C
Bronchioles terminate in alveolar sacs, which consist of clusters of alveoli. These alveoli are the true sites of gaseous exchange in the lungs.Incorrect
Explanation: D
1. Pathway of air in humans:
Trachea → Primary bronchi → Secondary bronchi → Tertiary bronchi → Bronchioles → Alveolar ducts → Alveolar sacs → Alveoli.
2. Alveoli are the tiny air sacs where gas exchange occurs.
3. Alveolar sacs are clusters (bunches) of alveoli at the ends of bronchioles.
4. So, bronchioles don’t end directly in a single alveolus but in alveolar sacs made up of many alveoli.
Correct Answer: D. Both B and C
Bronchioles terminate in alveolar sacs, which consist of clusters of alveoli. These alveoli are the true sites of gaseous exchange in the lungs. -
Question 81 of 100
81. Question
1 pointsBone is surrounded by a membrane called:
Correct
Explanation: D
1. Perichondrium → membrane covering cartilage, not bone
2. Prostomium → a part of the annelid worm’s head region, not related to bone
3. Perimysium → connective tissue surrounding bundles of muscle fibers (fascicles)
4. Periosteum → tough, vascular membrane covering the outer surface of bones
Correct Answer: D. Periosteum
The periosteum is a dense, fibrous, vascular membrane that surrounds bones (except at joint surfaces). It plays a key role in bone growth, repair, and nutrient supply.Incorrect
Explanation: D
1. Perichondrium → membrane covering cartilage, not bone
2. Prostomium → a part of the annelid worm’s head region, not related to bone
3. Perimysium → connective tissue surrounding bundles of muscle fibers (fascicles)
4. Periosteum → tough, vascular membrane covering the outer surface of bones
Correct Answer: D. Periosteum
The periosteum is a dense, fibrous, vascular membrane that surrounds bones (except at joint surfaces). It plays a key role in bone growth, repair, and nutrient supply. -
Question 82 of 100
82. Question
1 pointsSkull of a human is made up of:
Correct
Explanation: C
1. The human skull is made of 22 bones (excluding ear ossicles and hyoid):
• Cranial bones = 8 (frontal, 2 parietal, 2 temporal, occipital, sphenoid, ethmoid).
• Facial bones = 14 (maxilla, mandible, nasal, zygomatic, palatine, vomer, lacrimal, inferior nasal conchae).
2. If ear ossicles are included: add 6 bones (malleus, incus, stapes × 2).
3. That makes the total 28 bones (22 skull bones + 6 ossicles).
Correct Answer:
• Strictly speaking, 22 bones form the skull proper.
• If the examiner includes ear ossicles, then it’s 28 bones.
Most textbooks (especially at FSc level) state the skull is made up of 22 bones. However, in some contexts (especially anatomy), when middle ear ossicles are counted, the total becomes 28.Incorrect
Explanation: C
1. The human skull is made of 22 bones (excluding ear ossicles and hyoid):
• Cranial bones = 8 (frontal, 2 parietal, 2 temporal, occipital, sphenoid, ethmoid).
• Facial bones = 14 (maxilla, mandible, nasal, zygomatic, palatine, vomer, lacrimal, inferior nasal conchae).
2. If ear ossicles are included: add 6 bones (malleus, incus, stapes × 2).
3. That makes the total 28 bones (22 skull bones + 6 ossicles).
Correct Answer:
• Strictly speaking, 22 bones form the skull proper.
• If the examiner includes ear ossicles, then it’s 28 bones.
Most textbooks (especially at FSc level) state the skull is made up of 22 bones. However, in some contexts (especially anatomy), when middle ear ossicles are counted, the total becomes 28. -
Question 83 of 100
83. Question
1 pointsA wave of electrochemical change that travels along a neuron is called:
Correct
Explanation: C
1. A nerve impulse is an electrochemical wave caused by the movement of ions (Na+, K+) across the neuron’s membrane.
• It involves depolarization → repolarization → hyperpolarization.
• This is the actual signal traveling along the axon.
2. Check options:
• A. Reflex arc → pathway involving receptor, sensory neuron, interneuron, motor neuron, effector. It’s not the wave itself.
• B. Reflex action → automatic, involuntary response (e.g., knee jerk).
• C. Nerve impulse → correct term for the electrochemical wave.
• D. Influx → refers to entry of ions (like Na⁺ influx), part of the process but not the whole wave.
Correct Answer: C. Nerve impulse
A nerve impulse is the traveling wave of depolarization and repolarization along the neuron membrane, allowing transmission of information.Incorrect
Explanation: C
1. A nerve impulse is an electrochemical wave caused by the movement of ions (Na+, K+) across the neuron’s membrane.
• It involves depolarization → repolarization → hyperpolarization.
• This is the actual signal traveling along the axon.
2. Check options:
• A. Reflex arc → pathway involving receptor, sensory neuron, interneuron, motor neuron, effector. It’s not the wave itself.
• B. Reflex action → automatic, involuntary response (e.g., knee jerk).
• C. Nerve impulse → correct term for the electrochemical wave.
• D. Influx → refers to entry of ions (like Na⁺ influx), part of the process but not the whole wave.
Correct Answer: C. Nerve impulse
A nerve impulse is the traveling wave of depolarization and repolarization along the neuron membrane, allowing transmission of information. -
Question 84 of 100
84. Question
1 pointsThe type of nerve impulse in a non-myelinated neuron is:
Correct
Explanation: A
1. Impulse conduction in neurons:
• Myelinated neurons: Have myelin sheath → impulse “jumps” from node to node = saltatory conduction (fast).
• Non-myelinated neurons: Lack myelin sheath → impulse moves step by step along the entire membrane = continuous conduction (slower).
2. Check options:
• A. Continuous (Correct) → conduction is continuous along the axon.
• B. Stationary → impulse never stays still; it propagates.
• C. Electrical → impulse is electrochemical, not purely electrical.
• D. All of them → since only A is correct.
Correct Answer: A. Continuous
In non-myelinated neurons, the action potential spreads continuously along the membrane because there are no myelin gaps (nodes of Ranvier) to speed up conduction.Incorrect
Explanation: A
1. Impulse conduction in neurons:
• Myelinated neurons: Have myelin sheath → impulse “jumps” from node to node = saltatory conduction (fast).
• Non-myelinated neurons: Lack myelin sheath → impulse moves step by step along the entire membrane = continuous conduction (slower).
2. Check options:
• A. Continuous (Correct) → conduction is continuous along the axon.
• B. Stationary → impulse never stays still; it propagates.
• C. Electrical → impulse is electrochemical, not purely electrical.
• D. All of them → since only A is correct.
Correct Answer: A. Continuous
In non-myelinated neurons, the action potential spreads continuously along the membrane because there are no myelin gaps (nodes of Ranvier) to speed up conduction. -
Question 85 of 100
85. Question
1 pointsTestosterone, estrogen, and progesterone are hormones of:
Correct
Explanation: A
1. Classification of hormones by chemical nature:
• Steroid hormones: Derived from cholesterol (e.g., testosterone, estrogen, progesterone, cortisol, aldosterone).
• Amino acid derivatives: From tyrosine or tryptophan (e.g., thyroxine, adrenaline, melatonin).
• Protein/Polypeptide hormones: Chains of amino acids (e.g., insulin, glucagon, growth hormone).
2. Testosterone, estrogen, progesterone → all are sex hormones derived from cholesterol → hence steroid hormones.
3. Check options:
A. Steroid nature Correct.
B. Amino acid nature Wrong.
C. Protein nature Wrong.
D. Polypeptide nature Wrong.
Correct Answer: A. Steroid nature
Sex hormones such as testosterone, estrogen, and progesterone belong to the steroid family, synthesized from cholesterol, and are lipid-soluble. They pass through cell membranes and act via intracellular receptors to regulate gene expression.Incorrect
Explanation: A
1. Classification of hormones by chemical nature:
• Steroid hormones: Derived from cholesterol (e.g., testosterone, estrogen, progesterone, cortisol, aldosterone).
• Amino acid derivatives: From tyrosine or tryptophan (e.g., thyroxine, adrenaline, melatonin).
• Protein/Polypeptide hormones: Chains of amino acids (e.g., insulin, glucagon, growth hormone).
2. Testosterone, estrogen, progesterone → all are sex hormones derived from cholesterol → hence steroid hormones.
3. Check options:
A. Steroid nature Correct.
B. Amino acid nature Wrong.
C. Protein nature Wrong.
D. Polypeptide nature Wrong.
Correct Answer: A. Steroid nature
Sex hormones such as testosterone, estrogen, and progesterone belong to the steroid family, synthesized from cholesterol, and are lipid-soluble. They pass through cell membranes and act via intracellular receptors to regulate gene expression. -
Question 86 of 100
86. Question
1 pointsInsulin is secreted by:
Correct
Explanation: B
1. In the pancreas, the endocrine part is the Islets of Langerhans.
• Alpha cells → secrete glucagon (raises blood glucose).
• Beta cells → secrete insulin (lowers blood glucose).
• Delta cells → secrete somatostatin (inhibits both insulin & glucagon).
• Acinar cells → part of the exocrine pancreas, secreting digestive enzymes, not hormones.
2. Since insulin is secreted by beta cells, the correct option is B.
Correct Answer: B. Beta cells
Beta cells of the islets of Langerhans produce insulin, which decreases blood glucose by promoting glucose uptake into cells and conversion of glucose into glycogen (glycogenesis) in liver and muscles.Incorrect
Explanation: B
1. In the pancreas, the endocrine part is the Islets of Langerhans.
• Alpha cells → secrete glucagon (raises blood glucose).
• Beta cells → secrete insulin (lowers blood glucose).
• Delta cells → secrete somatostatin (inhibits both insulin & glucagon).
• Acinar cells → part of the exocrine pancreas, secreting digestive enzymes, not hormones.
2. Since insulin is secreted by beta cells, the correct option is B.
Correct Answer: B. Beta cells
Beta cells of the islets of Langerhans produce insulin, which decreases blood glucose by promoting glucose uptake into cells and conversion of glucose into glycogen (glycogenesis) in liver and muscles. -
Question 87 of 100
87. Question
1 pointsInsulin is manufactured commercially using genetic engineering. Which organism is used?
Correct
Explanation: B
1. Background:
• Before genetic engineering, diabetic patients were treated with animal insulin (from pigs or cattle).
• This caused some allergic reactions and was expensive.
2. Modern method:
• Scientists introduced the human insulin gene into plasmids (circular DNA) of E. coli bacteria using recombinant DNA technology.
• The bacteria multiply rapidly, producing large amounts of human insulin.
• The insulin is then extracted and purified for medical use.
3. Check options:
A. Viruses → Not used in commercial insulin production.
B. Bacteria (E. coli) Correct → main organism used in recombinant DNA technology for insulin.
C. Fungi → Some fungi are used for producing antibiotics (e.g., Penicillium), but not insulin.
D. Animals → Earlier source, but not modern genetic engineering method.
Correct Answer: B. Bacteria (E. coli)
In genetic engineering, the human insulin gene is inserted into a bacterial plasmid, and this recombinant plasmid is introduced into E. coli. As the bacteria multiply, they produce human insulin, which is harvested, purified, and sold under names like Humulin. This method provides a safe, cheap, and allergy-free source of insulin for diabetic patients.Incorrect
Explanation: B
1. Background:
• Before genetic engineering, diabetic patients were treated with animal insulin (from pigs or cattle).
• This caused some allergic reactions and was expensive.
2. Modern method:
• Scientists introduced the human insulin gene into plasmids (circular DNA) of E. coli bacteria using recombinant DNA technology.
• The bacteria multiply rapidly, producing large amounts of human insulin.
• The insulin is then extracted and purified for medical use.
3. Check options:
A. Viruses → Not used in commercial insulin production.
B. Bacteria (E. coli) Correct → main organism used in recombinant DNA technology for insulin.
C. Fungi → Some fungi are used for producing antibiotics (e.g., Penicillium), but not insulin.
D. Animals → Earlier source, but not modern genetic engineering method.
Correct Answer: B. Bacteria (E. coli)
In genetic engineering, the human insulin gene is inserted into a bacterial plasmid, and this recombinant plasmid is introduced into E. coli. As the bacteria multiply, they produce human insulin, which is harvested, purified, and sold under names like Humulin. This method provides a safe, cheap, and allergy-free source of insulin for diabetic patients. -
Question 88 of 100
88. Question
1 pointsWhich structures are involved in controlling body temperature?
Correct
Explanation: B
1. Thermoregulation in humans:
• Hypothalamus → the control center for temperature (“body’s thermostat”).
• Blood vessels (in skin) → vasodilation releases heat, vasoconstriction conserves heat.
• Sweat glands → sweating removes heat through evaporation.
• Skeletal muscles → shivering generates heat when body is cold.
2. Check options:
• A. Blood vessels + cerebellum + sweat glands Cerebellum controls coordination of movement, not temperature.
• B. Blood vessels + hypothalamus + skeletal muscles Correct → all three play a direct role in thermoregulation.
• C. Kidneys + cerebellum + sweat glands Kidneys regulate excretion and water balance, not body temperature.
• D. Kidneys + hypothalamus + skeletal muscles Kidneys not involved in temperature control.
Correct Answer: B. Blood vessels + hypothalamus + skeletal muscles
The hypothalamus detects temperature changes, blood vessels and sweat glands regulate heat loss, and skeletal muscles produce heat through shivering. This integrated system maintains homeostasis of body temperature.Incorrect
Explanation: B
1. Thermoregulation in humans:
• Hypothalamus → the control center for temperature (“body’s thermostat”).
• Blood vessels (in skin) → vasodilation releases heat, vasoconstriction conserves heat.
• Sweat glands → sweating removes heat through evaporation.
• Skeletal muscles → shivering generates heat when body is cold.
2. Check options:
• A. Blood vessels + cerebellum + sweat glands Cerebellum controls coordination of movement, not temperature.
• B. Blood vessels + hypothalamus + skeletal muscles Correct → all three play a direct role in thermoregulation.
• C. Kidneys + cerebellum + sweat glands Kidneys regulate excretion and water balance, not body temperature.
• D. Kidneys + hypothalamus + skeletal muscles Kidneys not involved in temperature control.
Correct Answer: B. Blood vessels + hypothalamus + skeletal muscles
The hypothalamus detects temperature changes, blood vessels and sweat glands regulate heat loss, and skeletal muscles produce heat through shivering. This integrated system maintains homeostasis of body temperature. -
Question 89 of 100
89. Question
1 pointsWhich statement is correct for all viruses but not all bacteria/fungi?
Correct
Explanation: C
1. Viruses:
• Non-cellular, no cytoplasm, no nucleus, no organelles.
• Genetic material is either DNA or RNA (never both), enclosed in a protein coat (capsid).
• They cannot reproduce independently → must infect a host → obligate parasites.
2. Check options:
A. Most of cell is cytoplasm Viruses have no cytoplasm at all.
B. Outer layer is cellulose Found in plant cell walls and some fungi, not in viruses.
C. They are all parasites (Correct)→ all viruses are obligate intracellular parasites, while many bacteria/fungi can be free-living.
D. They have a nucleus containing DNA or RNA Viruses don’t have a nucleus; they only have nucleic acid + capsid.
Correct Answer: C. They are all parasites
Unlike bacteria and fungi (many of which are free-living), all viruses are obligate parasites, meaning they can only replicate inside living host cells. This is the key feature distinguishing viruses from cellular life forms.Incorrect
Explanation: C
1. Viruses:
• Non-cellular, no cytoplasm, no nucleus, no organelles.
• Genetic material is either DNA or RNA (never both), enclosed in a protein coat (capsid).
• They cannot reproduce independently → must infect a host → obligate parasites.
2. Check options:
A. Most of cell is cytoplasm Viruses have no cytoplasm at all.
B. Outer layer is cellulose Found in plant cell walls and some fungi, not in viruses.
C. They are all parasites (Correct)→ all viruses are obligate intracellular parasites, while many bacteria/fungi can be free-living.
D. They have a nucleus containing DNA or RNA Viruses don’t have a nucleus; they only have nucleic acid + capsid.
Correct Answer: C. They are all parasites
Unlike bacteria and fungi (many of which are free-living), all viruses are obligate parasites, meaning they can only replicate inside living host cells. This is the key feature distinguishing viruses from cellular life forms. -
Question 90 of 100
90. Question
1 pointsHow many CO2 molecules from 2 glucose molecules in aerobic respiration?
Correct
Explanation: D
1. One glucose molecule (C6H12O6) in aerobic respiration:
• Glycolysis: produces 2 pyruvate (3C each).
• Link reaction (pyruvate → acetyl-CoA): each pyruvate (2 per glucose) produces 1 CO2 → total 2 CO2.
• Krebs cycle: each acetyl-CoA (2 per glucose) produces 2 CO2 → total 4 CO2.
• Total per glucose = 2 + 4 = 6 CO2.
2. For 2 glucose molecules:
• 6 × 2 = 126 × 2 = 12 CO2.
Correct Answer: D. 12
In aerobic respiration, each glucose molecule is completely oxidized into 6 CO2 molecules. Therefore, for 2 glucose molecules, the total CO2 released is 12 molecules.Incorrect
Explanation: D
1. One glucose molecule (C6H12O6) in aerobic respiration:
• Glycolysis: produces 2 pyruvate (3C each).
• Link reaction (pyruvate → acetyl-CoA): each pyruvate (2 per glucose) produces 1 CO2 → total 2 CO2.
• Krebs cycle: each acetyl-CoA (2 per glucose) produces 2 CO2 → total 4 CO2.
• Total per glucose = 2 + 4 = 6 CO2.
2. For 2 glucose molecules:
• 6 × 2 = 126 × 2 = 12 CO2.
Correct Answer: D. 12
In aerobic respiration, each glucose molecule is completely oxidized into 6 CO2 molecules. Therefore, for 2 glucose molecules, the total CO2 released is 12 molecules. -
Question 91 of 100
91. Question
1 pointsWhich statement about chromosomes is correct?
Correct
Explanation: A
1. What is a chromosome?
• A chromosome is a highly coiled structure made of DNA + proteins (histones).
• Each chromosome contains a long, continuous DNA molecule.
• The DNA is functionally divided into genes (units of heredity).
2. Check options:
• A. Chromosomes are long DNA molecules divided into genes Correct. That’s the precise definition.
• B. Chromosomes include a long molecule of DNA divided into genes → Sounds close, but “include” is vague; the chromosome is the DNA molecule + proteins, not something that “includes” it. Less precise.
• C. Genes include DNA molecules called chromosomes Wrong. Genes are segments of DNA, not containers of chromosomes.
• D. Genes are long DNA molecules called chromosomes Wrong. Genes are small sections of DNA, not whole chromosomes.
Correct Answer: A. Chromosomes are long DNA molecules divided into genes
Chromosomes are thread-like structures carrying a single long DNA molecule, and this DNA is functionally divided into genes. Genes are the specific segments that code for proteins or functional RNAs.Incorrect
Explanation: A
1. What is a chromosome?
• A chromosome is a highly coiled structure made of DNA + proteins (histones).
• Each chromosome contains a long, continuous DNA molecule.
• The DNA is functionally divided into genes (units of heredity).
2. Check options:
• A. Chromosomes are long DNA molecules divided into genes Correct. That’s the precise definition.
• B. Chromosomes include a long molecule of DNA divided into genes → Sounds close, but “include” is vague; the chromosome is the DNA molecule + proteins, not something that “includes” it. Less precise.
• C. Genes include DNA molecules called chromosomes Wrong. Genes are segments of DNA, not containers of chromosomes.
• D. Genes are long DNA molecules called chromosomes Wrong. Genes are small sections of DNA, not whole chromosomes.
Correct Answer: A. Chromosomes are long DNA molecules divided into genes
Chromosomes are thread-like structures carrying a single long DNA molecule, and this DNA is functionally divided into genes. Genes are the specific segments that code for proteins or functional RNAs. -
Question 92 of 100
92. Question
1 pointsGenotype Tt for height. What conclusion?
Correct
Explanation: B
1. Genotype Tt meaning:
• T = dominant allele (tallness).
• t = recessive allele (shortness).
• Together, Tt shows the organism is heterozygous for the height gene.
2. Check options:
A. Allele has at least 2 genes (Wrong). An allele is a version of a single gene, not multiple genes.
B. There are at least two different alleles of the gene (Correct). The genotype shows that the height gene exists in two forms: T and t.
C. Two different genes for height (Wrong). Only one gene for height, but it has two alleles.
D. One allele with 2 forms Slightly misleading. It’s the gene that has two forms (alleles), not the allele itself.
Correct Answer: B. There are at least two different alleles of the gene
Genotype Tt proves that the height gene exists in more than one form (alleles). The capital T (dominant) codes for tallness, and small t (recessive) codes for shortness. Having Tt shows heterozygosity → two different alleles for the same gene.Incorrect
Explanation: B
1. Genotype Tt meaning:
• T = dominant allele (tallness).
• t = recessive allele (shortness).
• Together, Tt shows the organism is heterozygous for the height gene.
2. Check options:
A. Allele has at least 2 genes (Wrong). An allele is a version of a single gene, not multiple genes.
B. There are at least two different alleles of the gene (Correct). The genotype shows that the height gene exists in two forms: T and t.
C. Two different genes for height (Wrong). Only one gene for height, but it has two alleles.
D. One allele with 2 forms Slightly misleading. It’s the gene that has two forms (alleles), not the allele itself.
Correct Answer: B. There are at least two different alleles of the gene
Genotype Tt proves that the height gene exists in more than one form (alleles). The capital T (dominant) codes for tallness, and small t (recessive) codes for shortness. Having Tt shows heterozygosity → two different alleles for the same gene. -
Question 93 of 100
93. Question
1 pointsIn human circulatory system, what causes transfer from capillaries to tissue fluid?
Correct
Explanation: B
1. In capillaries, substances move between blood and tissue fluid
2. At the arterial end of capillary, blood pressure (hydrostatic pressure) is high → pushes water, oxygen, glucose out into tissue fluid.
3. At the venous end of capillary, osmotic pressure (due to plasma proteins) is higher → pulls water back in.
4. So, the initial transfer into tissue fluid is mainly due to blood pressure.
Correct Answer: B. Blood pressure
The force of blood pressure at the arterial end of capillaries forces fluid out, forming tissue fluid. Osmosis plays a role later in reabsorption, but the key factor for transfer from capillaries to tissues is blood pressure.Incorrect
Explanation: B
1. In capillaries, substances move between blood and tissue fluid
2. At the arterial end of capillary, blood pressure (hydrostatic pressure) is high → pushes water, oxygen, glucose out into tissue fluid.
3. At the venous end of capillary, osmotic pressure (due to plasma proteins) is higher → pulls water back in.
4. So, the initial transfer into tissue fluid is mainly due to blood pressure.
Correct Answer: B. Blood pressure
The force of blood pressure at the arterial end of capillaries forces fluid out, forming tissue fluid. Osmosis plays a role later in reabsorption, but the key factor for transfer from capillaries to tissues is blood pressure. -
Question 94 of 100
94. Question
1 pointsWhy is right ventricle wall thicker than right atrium?
Correct
Explanation: C
1. Right atrium → receives blood from vena cavae and pushes it only into the right ventricle → requires little force → thin wall.
2. Right ventricle → pumps blood into the lungs through pulmonary artery → requires more pressure than the atrium (though less than the left ventricle, which pumps to the whole body).
3. Wall thickness is due to the force required for pumping, not O₂ concentration or blood volume.
Correct Answer: C. Right ventricle must exert greater force
The ventricle walls are thicker than atria because ventricles must generate more pressure to pump blood out of the heart (to lungs or body), whereas atria only push blood into nearby ventricles.Incorrect
Explanation: C
1. Right atrium → receives blood from vena cavae and pushes it only into the right ventricle → requires little force → thin wall.
2. Right ventricle → pumps blood into the lungs through pulmonary artery → requires more pressure than the atrium (though less than the left ventricle, which pumps to the whole body).
3. Wall thickness is due to the force required for pumping, not O₂ concentration or blood volume.
Correct Answer: C. Right ventricle must exert greater force
The ventricle walls are thicker than atria because ventricles must generate more pressure to pump blood out of the heart (to lungs or body), whereas atria only push blood into nearby ventricles. -
Question 95 of 100
95. Question
1 pointsIn dialysis machine, which substance will not diffuse into dialysis fluid?
Correct
Explanation: A
1. Dialysis principle:
• Blood is passed through a semi-permeable membrane.
• Small waste molecules (e.g., urea, salts, excess water) diffuse into dialysis fluid.
• Dialysis fluid has a controlled concentration to maintain correct salt and water balance.
2. Proteins:
• Large molecules → cannot pass through dialysis membrane.
• They remain in blood and do not diffuse into dialysis fluid.
3. Check options:
A. Protein Too large to diffuse → correct.
B. Salts Small ions diffuse until balanced.
C. Urea Small waste product → diffuses out.
D. Water Can move by osmosis through membrane.
Correct Answer: A. Protein
Dialysis removes urea, salts, and excess water but retains proteins and blood cells because they are too large to pass through the semi-permeable membrane.Incorrect
Explanation: A
1. Dialysis principle:
• Blood is passed through a semi-permeable membrane.
• Small waste molecules (e.g., urea, salts, excess water) diffuse into dialysis fluid.
• Dialysis fluid has a controlled concentration to maintain correct salt and water balance.
2. Proteins:
• Large molecules → cannot pass through dialysis membrane.
• They remain in blood and do not diffuse into dialysis fluid.
3. Check options:
A. Protein Too large to diffuse → correct.
B. Salts Small ions diffuse until balanced.
C. Urea Small waste product → diffuses out.
D. Water Can move by osmosis through membrane.
Correct Answer: A. Protein
Dialysis removes urea, salts, and excess water but retains proteins and blood cells because they are too large to pass through the semi-permeable membrane. -
Question 96 of 100
96. Question
1 pointsCausative organism of syphilis?
Correct
Explanation: A
1. Syphilis → a sexually transmitted disease (STD) caused by a spirochete bacterium.
• The bacterium is Treponema pallidum.
2. Check options:
A. Treponema pallidum Correct.
B. Mycoplasma pneumoniae Causes atypical pneumonia.
C. Plasmodium Protozoan causing malaria.
D. Vibrio cholerae Bacterium causing cholera.
Correct Answer: A. Treponema pallidum
Syphilis is caused by the spirochete bacterium Treponema pallidum, transmitted mainly through sexual contact, from mother to fetus (congenital syphilis), or less commonly through blood transfusion.Incorrect
Explanation: A
1. Syphilis → a sexually transmitted disease (STD) caused by a spirochete bacterium.
• The bacterium is Treponema pallidum.
2. Check options:
A. Treponema pallidum Correct.
B. Mycoplasma pneumoniae Causes atypical pneumonia.
C. Plasmodium Protozoan causing malaria.
D. Vibrio cholerae Bacterium causing cholera.
Correct Answer: A. Treponema pallidum
Syphilis is caused by the spirochete bacterium Treponema pallidum, transmitted mainly through sexual contact, from mother to fetus (congenital syphilis), or less commonly through blood transfusion. -
Question 97 of 100
97. Question
1 pointsWhich immunoglobulin provides long-term immunity after vaccination?
Correct
Explanation: B
1. Immunoglobulin types and roles:
• IgA → found in secretions (tears, saliva, mucus, breast milk) → mucosal protection.
• IgM → first antibody produced in primary response → short-lived.
• IgE → involved in allergic reactions and defense against parasites.
• IgG → most abundant antibody in blood, produced in secondary immune response, provides long-term immunity after infection or vaccination.
2. Vaccination → mimics infection → stimulates immune system → memory B-cells → secondary response produces IgG for long-term protection.
Correct Answer: B. IgG
After vaccination, IgM is produced first, but it is replaced by IgG, which remains in circulation for years, providing long-term immunity and rapid response upon re-exposure.Incorrect
Explanation: B
1. Immunoglobulin types and roles:
• IgA → found in secretions (tears, saliva, mucus, breast milk) → mucosal protection.
• IgM → first antibody produced in primary response → short-lived.
• IgE → involved in allergic reactions and defense against parasites.
• IgG → most abundant antibody in blood, produced in secondary immune response, provides long-term immunity after infection or vaccination.
2. Vaccination → mimics infection → stimulates immune system → memory B-cells → secondary response produces IgG for long-term protection.
Correct Answer: B. IgG
After vaccination, IgM is produced first, but it is replaced by IgG, which remains in circulation for years, providing long-term immunity and rapid response upon re-exposure. -
Question 98 of 100
98. Question
1 pointsWhy is hemophilia more common in males?
Correct
Explanation: C
1. Hemophilia = genetic disorder where blood doesn’t clot properly due to deficiency of clotting factors (Hemophilia A = Factor VIII deficiency, Hemophilia B = Factor IX deficiency).
2. It is not autosomal, but sex-linked.
3. The defective gene is located on the X chromosome, inherited in an X-linked recessive manner.
• Males have only one X chromosome (XY) → if that X carries the mutation → disease appears.
• Females have two X chromosomes (XX) → if one X is defective, the other usually compensates → they are carriers but rarely affected.
4. Other options:
A. Dominant autosomal gene Not true.
B. Y-linked disorder Wrong; hemophilia gene is not on Y.
C. Inherited as X-linked recessive trait Correct.
D. Acquired due to liver disease Liver disease can cause clotting issues but not hereditary hemophilia.
Correct Answer: C. Inherited as X-linked recessive trait
Hemophilia is common in males because they have only one X chromosome. If it carries the recessive defective allele, there is no backup copy (unlike females), so the disorder appearsIncorrect
Explanation: C
1. Hemophilia = genetic disorder where blood doesn’t clot properly due to deficiency of clotting factors (Hemophilia A = Factor VIII deficiency, Hemophilia B = Factor IX deficiency).
2. It is not autosomal, but sex-linked.
3. The defective gene is located on the X chromosome, inherited in an X-linked recessive manner.
• Males have only one X chromosome (XY) → if that X carries the mutation → disease appears.
• Females have two X chromosomes (XX) → if one X is defective, the other usually compensates → they are carriers but rarely affected.
4. Other options:
A. Dominant autosomal gene Not true.
B. Y-linked disorder Wrong; hemophilia gene is not on Y.
C. Inherited as X-linked recessive trait Correct.
D. Acquired due to liver disease Liver disease can cause clotting issues but not hereditary hemophilia.
Correct Answer: C. Inherited as X-linked recessive trait
Hemophilia is common in males because they have only one X chromosome. If it carries the recessive defective allele, there is no backup copy (unlike females), so the disorder appears -
Question 99 of 100
99. Question
1 pointsCompared with days before ovulation, which hormone increases 3 days after ovulation?
Correct
Explanation: D
1. Ovulation timing: occurs around day 14 of a 28-day cycle, triggered by the LH surge.
2. After ovulation (luteal phase):
• The ruptured follicle transforms into the corpus luteum.
• The corpus luteum secretes large amounts of progesterone and some oestrogen.
• Progesterone prepares the endometrium for possible implantation.
3. Check options:
A. FSH Highest before ovulation to stimulate follicle growth, then decreases.
B. LH Surge occurs at ovulation, then drops quickly.
C. Oestrogen Peaks before ovulation, then shows only a slight rise again, not the main hormone after ovulation.
D. Progesterone This is the hormone that increases significantly after ovulation.
Correct Answer: D. Progesterone
Three days after ovulation (early luteal phase), progesterone levels rise sharply due to secretion from the corpus luteum. This differentiates the luteal phase from the follicular phase.Incorrect
Explanation: D
1. Ovulation timing: occurs around day 14 of a 28-day cycle, triggered by the LH surge.
2. After ovulation (luteal phase):
• The ruptured follicle transforms into the corpus luteum.
• The corpus luteum secretes large amounts of progesterone and some oestrogen.
• Progesterone prepares the endometrium for possible implantation.
3. Check options:
A. FSH Highest before ovulation to stimulate follicle growth, then decreases.
B. LH Surge occurs at ovulation, then drops quickly.
C. Oestrogen Peaks before ovulation, then shows only a slight rise again, not the main hormone after ovulation.
D. Progesterone This is the hormone that increases significantly after ovulation.
Correct Answer: D. Progesterone
Three days after ovulation (early luteal phase), progesterone levels rise sharply due to secretion from the corpus luteum. This differentiates the luteal phase from the follicular phase. -
Question 100 of 100
100. Question
1 pointsEndometriosis will cause infertility in:
Correct
Explanation: B
1. What is endometriosis?
• A condition where endometrial tissue (lining of uterus) grows outside the uterus (e.g., on ovaries, fallopian tubes, pelvic lining).
• This causes inflammation, scar tissue, blocked fallopian tubes, and disturbed ovulation.
2. Who does it affect?
• Only females, because endometrium is a female reproductive tissue.
• Males do not have endometrium, so they cannot get endometriosis.
3. Check options:
A. Male
B. Female (Correct).
C. Both Wrong, only females affected.
D. None Incorrect, as endometriosis clearly causes infertility in females.
Correct Answer: B. Female
Endometriosis can cause infertility in females by blocking fallopian tubes, disrupting ovulation, or damaging reproductive tissues.Incorrect
Explanation: B
1. What is endometriosis?
• A condition where endometrial tissue (lining of uterus) grows outside the uterus (e.g., on ovaries, fallopian tubes, pelvic lining).
• This causes inflammation, scar tissue, blocked fallopian tubes, and disturbed ovulation.
2. Who does it affect?
• Only females, because endometrium is a female reproductive tissue.
• Males do not have endometrium, so they cannot get endometriosis.
3. Check options:
A. Male
B. Female (Correct).
C. Both Wrong, only females affected.
D. None Incorrect, as endometriosis clearly causes infertility in females.
Correct Answer: B. Female
Endometriosis can cause infertility in females by blocking fallopian tubes, disrupting ovulation, or damaging reproductive tissues.